1 Introduction
In recent years, interest is increasing in harnessing sunlight energy to address energy-resource problems due to clean and inexhaustible supply. In order to use sunlight energy effectively, the design and development of photocatalytic systems capable of operating under visible or solar light irradiation have been desired for the applications of photocatalytic system especially to the environmental concerns. Recently, we have found that the metal-ion implantation of TiO2 with highly accelerated transition metal ions is useful to design photocatalysts that can operate efficiently under visible light irradiation [1,2]. It has been desired earnestly that the photocatalysts operatable under visible light irradiation can be prepared by the conventional chemical processes.
The highly dispersed transition metal oxides incorporated within the framework of zeolites and molecular sieves show unique reactivities not only for various catalytic reactions, but also for photocatalytic reactions under UV light irradiation [3–5]. Recently, unique and efficient photocatalytic systems incorporating the transition metal oxides (Ti, V, Mo, etc.) have been designed and developed using the cavities and frameworks of zeolites and mesoporous molecular sieves [6–8]. However, these metal oxides can operate as efficient photocatalysts only under UV light irradiation but exhibit no photocatalytic reactivity under visible light irradiation. Tetrahedrally-coordinated chromium oxide moieties (Cr-oxide) which are highly dispersed and incorporated in silica or zeolite show unique photocatalytic reactivity not only under UV light irradiation but also under visible-light irradiation.
In the present study, we have found that the Cr-oxide loaded on zeolites and mesoporous molecular sieves (HMS) can adsorb and utilize the visible light in the photocatalytic reactions such as the partial oxidation of propane with O2. The characterization of the local structure of the active sites and their role in the photocatalytic reaction have been investigated at the molecular level by means of dynamic photoluminescence, XAFS, UV–VIS, and XRD measurements along with an analysis of the reaction products.
2 Experimental
The photocatalysts, imp-Cr/HMS (Si/Cr = 100), imp-Cr/Y zeolite (Si/Cr = 100, SiO2/Al2O3 = 260) and imp-Cr/ZSM-5 zeolite (Si/Cr = 100, SiO2/Al2O3 = 1880) were prepared by impregnating HMS mesoporous silica [9,10], Y-zeolite and ZSM-5, respectively, with an aqueous solution of Cr(NO3)3·9 H2O. Calcination of the sample was carried out in a flow of dry air at 773 K for 5 h. Prior to spectroscopic measurements and photocatalytic reactions, the catalysts were degassed at 723 K for 2 h, heated in O2 at the same temperature for 2 h and then finally evacuated at 473 K for 2 h to 10−6 torr.
The photocatalytic reactions were carried out with the catalysts (50 mg) in a quartz cell with a flat bottom (80 ml) connected to a conventional vacuum system (10−6 torr range). The photocatalytic reactions we carried out under visible light (λ > 450 nm) irradiation at 313 K using a high pressure mercury lamp through water and color filters. The photocatalytic oxidation of propane with O2 was carried out in the presence of propane (317 μmol g−1) and O2 (634 μmol.g−1) and products in the gas phase and the products desorbed by heating to 573 K were analyzed by GC.
The in situ photoluminescence spectra the catalysts were measured at 77 K with a Shimadzu RF-501 spectrofluorophotometer. XAFS (XANES and EXAFS) spectra were obtained at the BL-9A facility of the Photon Factory at the National Laboratory for High Energy Physics (KEK-PF) in Tsukuba. The Cr K-edge absorption spectra were recorded in the fluorescence mode at 295 K with a ring energy of 2.5 GeV and the Fourier transformation was performed on k3-weighted EXAFS oscillations by a procedure described in previous literature [11–13]. UV–VIS spectra were recorded at 295 K with a Shimadzu UV-2400A spectrophotometer.
3 Results and discussion
Fig. 1 shows the XAFS spectra of the treated (a) Cr2O3, (b) CrO3, (c) K2CrO4, (d) imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), (e) imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and (f) imp-Cr/HMS (Si/Cr = 100). The imp-Cr/HMS exhibits a sharp and intense preedge peak which is characteristic of Cr-oxide moieties in tetrahedral coordination having terminal Cr=O [11,12,14]. In the FT-EXAFS spectrum, only a single peak due to the neighboring oxygen atoms (Cr–O) can be observed, showing that Cr ions are highly dispersed in Cr-HMS. In the curve fitting analysis of the FT-EXAFS spectrum, the best fitting was obtained with two oxygen atoms (Cr = O) in the shorter atomic distance of 1.62 Å and two oxygen atoms (Cr–O) in the long distance of 1.75 Å. The imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260) exhibits a week preedge peak in the XANES spectra, and this XANES spectra is similar to that of Cr2O3. In the FT-EXAFS spectrum, the peak due to neighboring O atoms (Cr–O) of tetrahedrally-coordinated Cr-oxide moieties and the peak due to neighboring Cr atoms (Cr–O–Cr) of the aggregated Cr-oxide species can be observed together. But the peak due to neighboring O atoms (Cr–O) of tetrahedrally-coordinated Cr-oxide moieties is observed in the longer position than in imp-Cr/HMS. These result indicates that imp-Cr/Y consists of a mixture of the minor of tetrahedrally- and the major of octahedrally-coordinated Cr-oxide species (Cr2O3-like cluster) and the tetrahedrally-coordinated Cr-oxide species are in the form of closed-type. The imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880) exhibits a sharp and intense preedge peak which is characteristic of tetrahedrally-coordinated Cr-oxide moieties in the XANES spectra. In the FT-EXAFS spectrum, the peak due to the neighboring O atoms (Cr–O) of tetrahedrally-coordinated Cr-oxide moieties is observed in the longer position than in imp-Cr/HMS. This indicates that the imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880) consists of a mixture of the major of tetrahedrally- and the minor of octahedrally-coordinated Cr-oxide species (Cr2O3-like cluster) and tetrahedrally-coordinated Cr-oxide species are in the form of open-type.

XANES (A–F) and FT-EXAFS (a–f) spectra of (a) Cr2O3, (b) CrO3, (c) K2CrO4, (d) imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), (e) imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3= 260), and (f) imp-Cr/HMS (Si/Cr = 100).
Fig. 2 shows the diffuse reflectance UV–VIS absorption spectra of imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and imp-Cr/HMS (Si/Cr = 100). The imp-Cr/ZSM-5 catalysts and imp-Cr/Y catalysts exhibit four distinct absorption bands at around 270, 340–380, 470, 600–650 nm. The three absorption bands at around 270, 370, 480 nm can be assigned to the charge transfer from O2– to Cr6+ of the tetrahedrally-coordinated Cr-oxide moieties [15,16]. The absorption bands assigned to the absorption of the dichromate of Cr2O3 cluster can be observed above 550 nm, indicating that tetrahedrally-coordinated Cr-oxide moieties exist. In the case of imp-Cr/HMS (Si/Cr = 100), only three distinct absorption bands, at around 270, 350, 470 nm, assigned to the charge transfer from O2– to Cr6+ of the tetrahedrally-coordinated Cr-oxide moieties are observed. The absorption bands assigned to the absorption of the dichromate of Cr2O3 cluster cannot be observed above 550 nm, indicating that tetrahedrally-coordinated Cr-oxide moieties exist in the isolated state on imp-Cr/HMS.
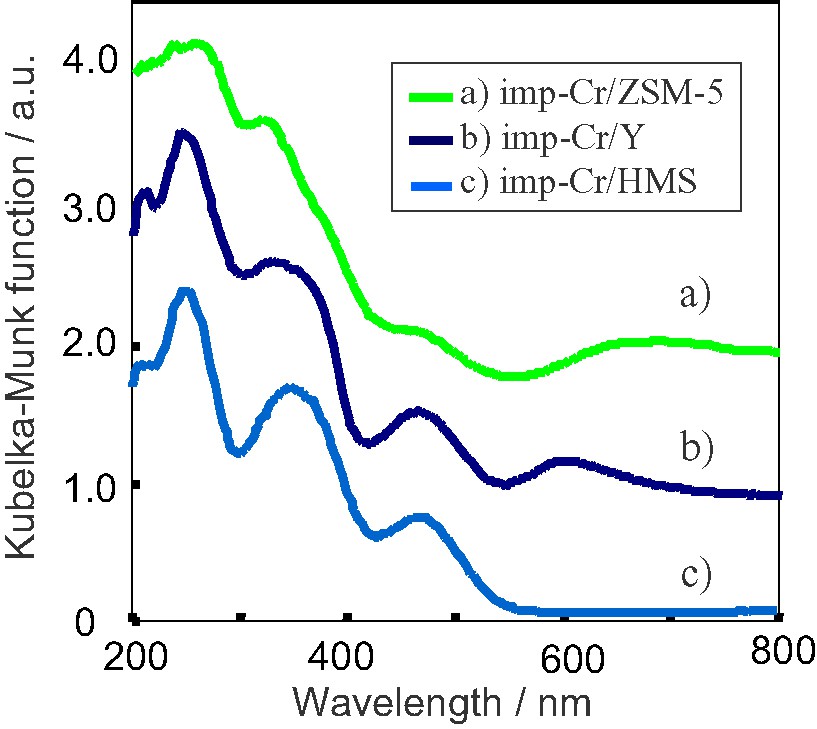
Diffuse reflectance UV–VIS spectra of (a) imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), (b) imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and (c) imp-Cr/HMS.
The imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and imp-Cr/HMS (Si/Cr = 100) evacuated at 473 K exhibited a photoluminescence spectrum at around 500–800 nm upon excitation of the absorption (excitation) band at around 250–550 nm. Fig. 3 shows the photoluminescence spectra of imp-Cr/ZSM-5, imp-Cr/Y, and imp-Cr/HMS observed at 77 K upon the excitation at 490, 510 and 510 nm, respectively. The photoluminescence upon excitation at around 270, 380, and 500 nm was observed at the same position, while the intensities of spectra depend on the wavelength of excitation. In the excitation spectrum of monitored at around 620 nm, three excitation bands are observed with the tetrahedrally-coordinated Cr-oxide species at around 270, 380, and 490 nm, which are corresponding to the absorption bands observed in the UV–VIS absorption spectra shown in Fig. 2. No change in the positions of these absorption bands is observed with changing the monitoring wavelength of photoluminescence. These results suggest that the photoluminescence occurs as the radiation decay process from the same excited state, independently to the excitation wavelength. These absorption and photoluminescence spectra are similar to those obtained with well-defined highly dispersed Cr-oxides anchored onto Vycor glass or silica [17–20] and can be attributed to the charge transfer processes on the tetrahedrally-coordinated Cr-oxide moieties involving an electron transfer from O2– to Cr6+ and a reverse radiative decay, respectively. These results indicate that the Cr-impregnated zeolites and mesoporous silica involves Cr-oxide moieties in tetrahedral coordination, being in agreement with the results obtained by XAFS measurements. The estimated model for the local structure of the Cr-oxide moieties and the charge transfer excited state are shown in Scheme 1.
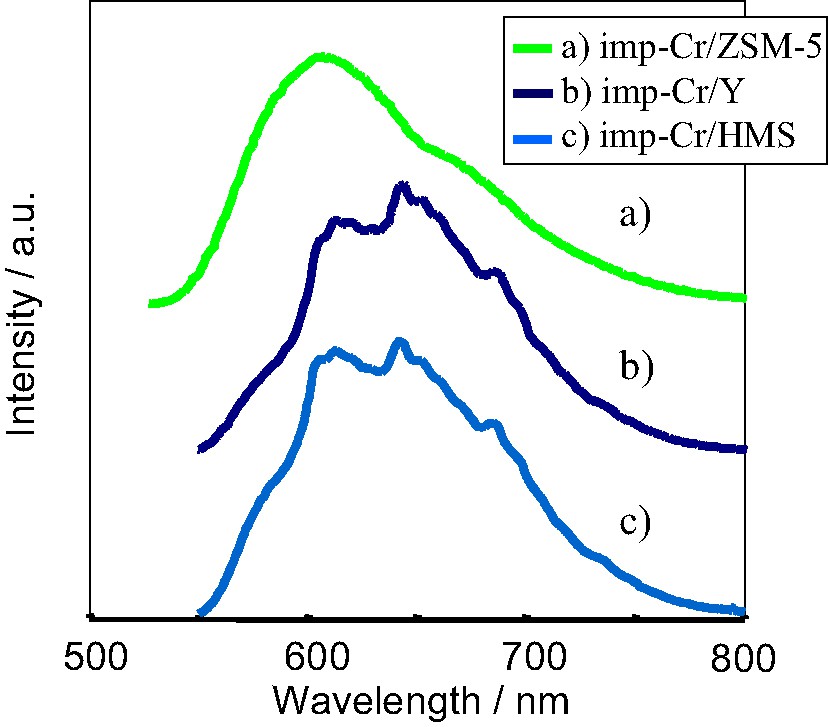
The photoluminescence spectra of (a) imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), (b) imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and (c) imp-Cr/HMS (Si/Cr = 100).
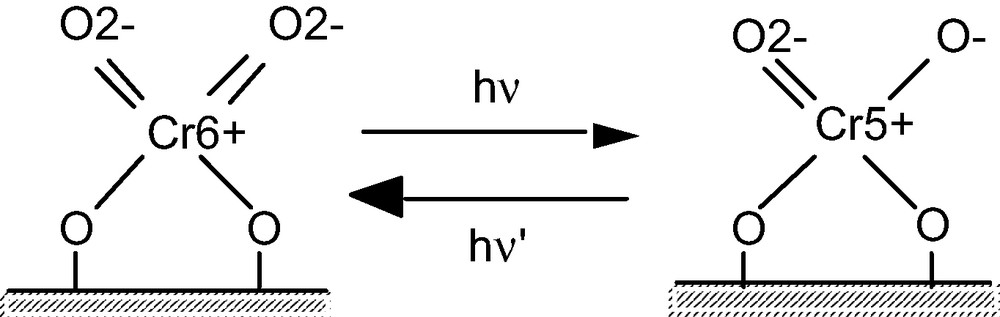
The charge transfer processes on the tetrahedrally-coordinated Cr-oxide moieties.
Visible-light irradiation of the imp-Cr/ZSM-5, imp-Cr/Y and the imp-Cr/HMS in the presence of propane and O2 led the photocatalytic oxidation of propane. Fig. 4. shows the comparison of the intensity of photoluminescence spectra under visible light irradiation among imp-Cr/HMS, imp-Cr/Y and imp-Cr/ZSM-5. The results of UV–VIS, photoluminescence and XAFS spectra suggest that local structure of Cr-oxide change depending on the pore size. The intensity of photoluminescence spectra decrease with the pore size becomes smaller.
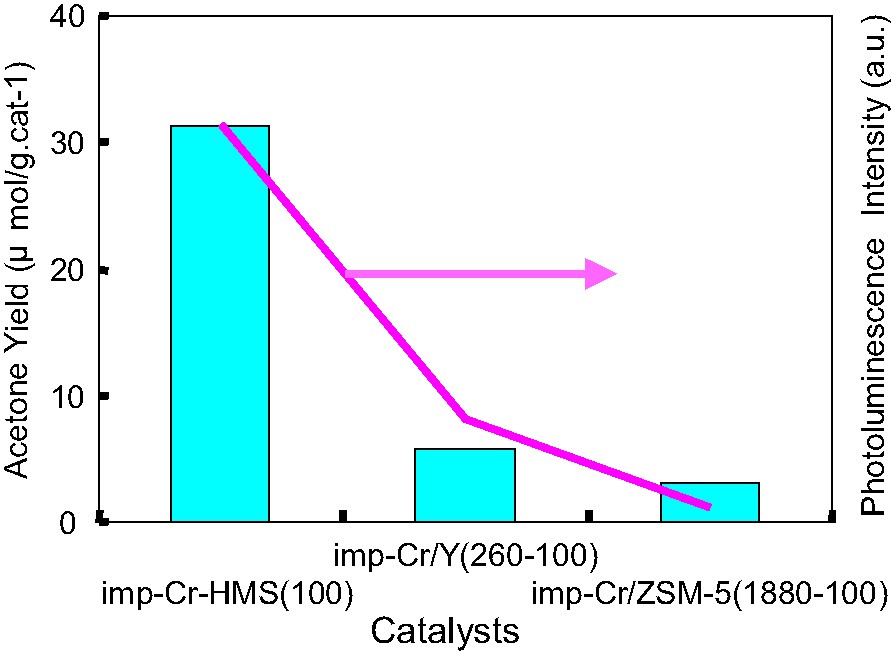
The intensity of photoluminescence and the yields of acetone in the photocatalytic oxidation of propane with O2 on imp-Cr/ZSM-5 (Si/Cr = 100, SiO2/Al2O3 = 1880), imp-Cr/Y (Si/Cr = 100, SiO2/Al2O3 = 260), and imp-Cr/HMS (Si/Cr = 100) under visible light irradiation (λ > 450 nm).
As shown in Fig. 4, partial oxidation of propane with a high selectivity for acetone formation proceeds with high intensity of photoluminescence. These results indicate that the tetrahedrally-coordinated isolated Cr-oxide moieties in mesoporous silica can exhibit the efficient photocatalytic reactivity for the oxidation of propane under visible light irradiation with a high selectivity for the partial oxidation of propane.
4 Conclusions
It has been found that imp-Cr/zeolite and mesoporous silica contained tetrahedrally-coordinated Cr-oxide moieties and that the charge transfer excited state of the Cr-oxide moieties are responsible for the efficient photoluminescence and photocatalytic reactivities. The present results have clearly demonstrated that the imp-Cr/zeolite and mesoporous silica can absorb visible light and act as an efficient and selective photocatalyst under visible light irradiation. This photocatalytic system with tetrahedrally-coordinated Cr-oxide moieties dispersed especially on mesoporous silica seems to be a good candidate to convert abundant visible or solar light energy into useful chemical energy.
Acknowledgements
This work is partly performed under the project of collaborative research at the Joining and Welding Research Institute (JWRI) of Osaka University. The X-ray adsorption experiments were performed at the Photon Factory of KEK (20003G251).


