1 Introduction
Fullerene dimers are attracting great attention not only because they can be assumed as models for fullerene polymers, but also because the extent of electronic communication between their cage subunits can constitute the first criterion towards their future application in molecular opto-electronics [1].
Apart from a fused dimeric fullerene [2], a number of dumb-bell-shaped fullerene dimers have been characterised in the last 15 years. Starting from directly C/C linked bisfullerenes [3], there are both organic- and inorganic-functionalised bisfullerenes. Concerned with organic dimers, they can be subdivided according to the nature of the connecting spacer: (i) carbon chains containing conjugated carbon/carbon bonds [4]; (ii) bridges containing heteroatoms in the carbon chain [5]; (iii) single carbon atom bridge [4h,6]; (iv) direct C/C connections enforced by bridging atoms [7].
As far as inorganic-bis-fullerenes are concerned, they can be subdivided depending upon if the interconnecting metal fragment contains [8] or lacks [5a,e,f,9] direct metal-fullerene bonding(s).
Stated that, from the electrochemical viewpoint, the eventual mutual interaction between the two fullerene units of bisfullerenes is proved by a more or less marked separation between the sequential electron additions to each single cage, organo-based bisfullerenes generally display very weak [3c,d,4a,6,7c,f,g] or no [4c,e–h,l,m,5c–g] interaction. In apparent contrast, a few inorganic bisfullerenes with direct metal-fullerene bondings, in particular those containing metal-carbonyl clusters as spacers [8b,c], unexpectedly look like to display rather strong electronic communication.
In this picture, we present here an electrochemical investigation on the quite symmetric [Mo(η2-C60)2(CO)2(dbc-bipy)] (dbc-bipy = 4,4′-di(butylcarboxyl)-2,2′-bipyridine) [8a] (Scheme 1).

2 Experimental
Preparation of [Mo(η2-C60)2(CO)2(dbc-bipy)] has been previously described [8a]. Material and apparatus for electrochemistry have been described elsewhere [10]. Potential values are referred to the Saturated Calomel Electrode (SCE). In CH2Cl2 solution containing [NBu4][PF6] (0.2 mol dm–3) as supporting electrolyte, the one-electron oxidation of ferrocene occurs at E°′ = +0.40 V. In the solution containing [NBu4][PF6] (0.2 mol dm–3) as supporting electrolyte, the one-electron oxidation of ferrocene occurs at E°′ = +0.52 V.
3 Results and discussion
Fig. 1 shows the cyclic voltammetric behaviour of [Mo(η2-C60)2(CO)2(dbc-bipy)] in CH2Cl2 solution.

Cyclic voltammetric responses recorded at a platinum electrode in CH2Cl2 solution of [Mo(η2-C60)2(CO)2(dbc-bipy)] (0.5 × 10–3 mol dm–3). [NBu4][PF6] (0.2 mol dm–3) supporting electrolyte. Scan rate: 0.2 V s–1.
It displays two first reductions exhibiting inflections in their profiles, followed by further single-stepped processes. Controlled potential coulometry in correspondence of the first cathodic process (Ew = –0.85 V) consumed 1.9 electrons per molecule, thus indicating that it involves two almost overlapping one-electron additions, which are confidently assigned as fullerene-centred. It cannot be ruled out that the two subsequent separated reductions (starred peaks in Fig. 1b) might also be fullerene centred, but considering that bipyridine ligands usually undergo reduction processes at rather negative potential values [11] and being impossible to ascertain their nature, we will no more dwell on them. A anodic process, not shown in figure, featuring partial chemical reversibility (ipc/ipa = 0.6 at 0.2 V s–1) is also present at E°′ = +1.06 V, which is assigned to the Mo-centred oxidation.
In order to better resolve the slightly separated stepwise reductions, a mathematical treatment has been carried out on both the cyclic voltammetric and Osteryoung square wave responses, first and second derivative modes in Figs. 2b and 3b, respectively.

First derivative profile (b) arising from the cyclic voltammetric response (a) of [Mo(η2-C60)2(CO)2(dbc-bipy)] recorded under the same experimental conditions as those of Fig. 1.
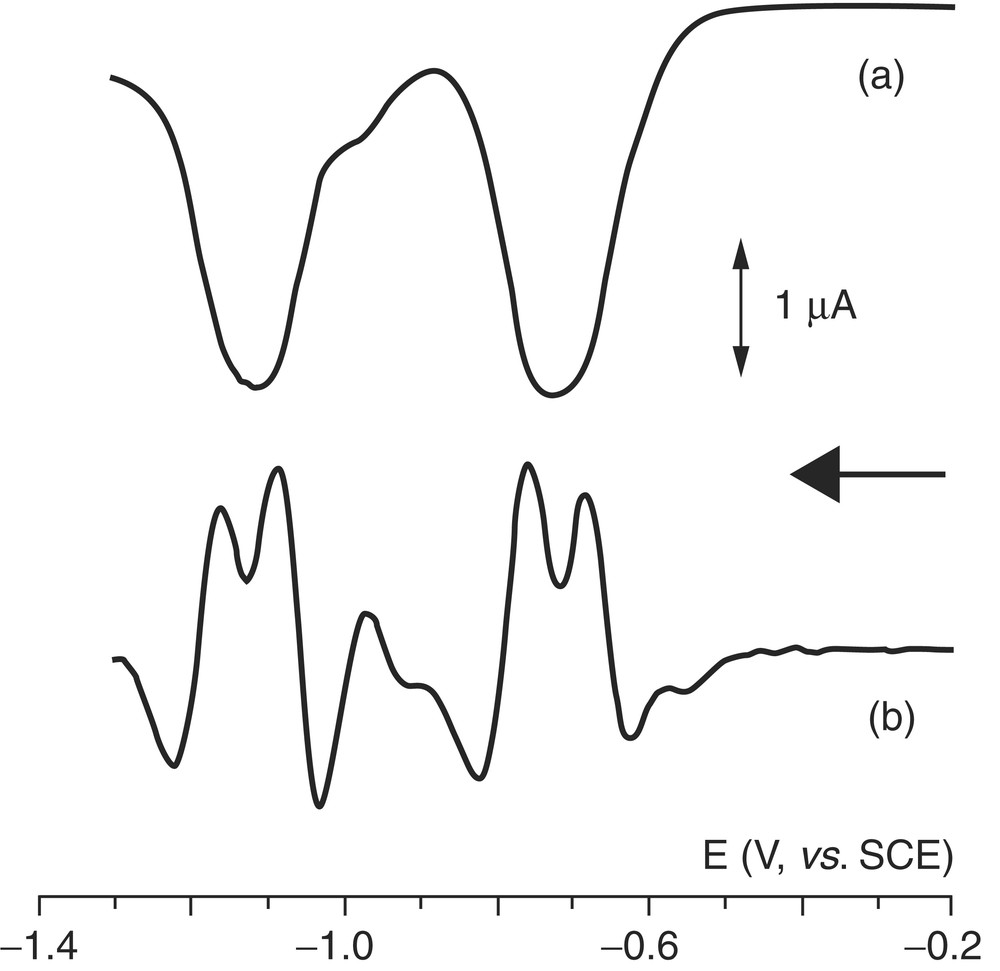
Second derivative profile (b) of the Osteryoung square-wave voltammetric response (a) of [Mo(η2-C60)2(CO)2(dbc-bipy)] recorded under the same experimental conditions as those of Fig. 1.
As a matter of fact, the first derivative treatment is not able to resolve adequately the two processes, even if applying the Taube's method for electrochemically reversible close-spaced cyclic voltammetric profiles [12], one could estimate for a separation of about 80 and 90 mV, respectively, for the two almost overlapped reductions.
In contrast, the second derivative treatment well resolves the two processes. The pertinent electrode potentials are compiled in Table 1, which also reports data in THF solution, which will be discussed later.
Formal electrode potentials (V, vs. SCE) for the sequential electron additions to [Mo(η2-C60)2(CO)2(dbc-bipy)] ([Mo(C60)2]) in non-aqueous solutions. The corresponding electron additions to C60 are also reported
| Complex | E°′0/− | E°−/2− | E°2−/3− | E°3−/4− | E°4−/5− | E°5−/6− | Solvent |
| [Mo(C60)2] | −0.69 a | −0.76 a | −1.09 a | −1.17 a | −1.49 | −1.7 b | CH2Cl2 |
| −0.41 b,c | −0.51 b,c | −0.94 b | −1.11 b,c | −1.39 b,c | −1.66 b,c | THF | |
| C60 | −0.58 | −0.96 | −1.42 | CH2Cl2 | |||
| −0.36 | −0.85 | −1.39 | THF |
a From second derivative response (see text).
b From differential pulse voltammetry.
c At –25 °C.
As happens in the case of a few organic-bisfullerenes [6,7c,f,g], because of coulombic repulsions, on passing from the almost simultaneous first one-electron to the successive electron additions to each cage, the wave splitting tends to increase up to give rise to substantially separate additions at the third step. Such a trend also holds for the metallo-bisfullerenes [Rh6(CO)5(dppm)2(CNCH2C6H5)(μ3-η2,η2,η2-C60)2] [8b] and Ir4(CO)3(μ4-CH)(PMe3)2(μ-PMe2)(CNCH2C6H5)(μ-η2,η2-C60)(μ4-η1,η1,η2,η2-C60) [8c].
As Table 2 compiles, assuming that the separation between the stepwise reductions might reflect the electronic interaction between the two cages, the metallo-bisfullerene under study exhibits an intramolecular interaction similar to that of most interacting organic fullerene dimers.
Separation (mV) between the stepwise reductions of different organic and inorganic fullerene dimers
| Complex | ΔEp(1st red) | ΔEp(2nd red) | ΔEp(3rd red) | Solvent | Reference |
| C120 | ≈ 80 a | – | – | C6H4Cl2 | [3d] |
| C121 | 80 | 96 | 172 | C6H4Cl2 | [6] |
| C120O | 39 | 61 | 138 | C6H4Cl2 | [7c] |
| C122H4 | ≈ 70 | ≈ 75 | ≈ 90 | C6H4Cl2 | [7f] |
| C120SiPh2 | 90 | 80 | 140 | C6H4Cl2 | [7g] |
| [C120Rh6] b | 190 | 240 | 290 | C6H5Cl | [8b] |
| [C120Ir4] b | 70 | 160 | 230 | C6H5Cl | [8c] |
| [Mo(C60)2] b | 70 | 80 | c | CH2Cl2 | d |
| 100 | 170 | c | THF | d |
a Followed by fast dissociation.
b For the correct formulation, see text.
c Not determined (see text).
d Present work.
In the case of the Rh6- and Ir4-inorganic-bisfullerenes, which apparently show a stronger interaction, it cannot be neglected that in both complexes the coordination of the interposed metal clusters to each fullerene moiety is not completely equivalent. This implies that each fullerene subunit could undergo reduction at different potential values, thus contributing to the separation of the stepwise reductions.
In the attempt to trace the through-space or through-bond nature of the mutual electronic interaction of the two fullerene subunits, it seemed us that a change of solvent could be of help. On this basis, we investigated the electrochemical behaviour of [Mo(η2-C60)2(CO)2(dbc-bipy)] also in THF solution. Since at room temperature the original complex proved to be not quite stable towards free C60 release, we examined it at −25 °C. Fig. 4 shows the resulting differential pulse voltammetric profile, also in comparison with that of free fullerene.

Differential pulse voltammetric responses recorded at a platinum electrode in THF solution of: (——) [Mo(η2-C60)2(CO)2(dbc-bipy)] (0.5 × 10–3 mol dm–3); (- - - -) C60 (0.4 × 10–3 mol dm–3). [NBu4][PF6] (0.2 mol dm–3) supporting electrolyte. T = –25 °C. Scan rate: 0.02 V s–1.
As seen, apart from traces of free fullerene (which increase as a consequence of the first electron additions) in this case no mathematical treatment is required to resolve the stepwise reductions. The pertinent formal electrode potentials are compiled in Table 1.
It hence results that, even if CH2Cl2 and THF do not differ greatly in dielectric constant (THF: ɛ = 7.4; CH2Cl2: ɛ = 8.9), the polarity change seems sufficient to affect the degree of separation of the sequential reductions, thus lending support to a through-space interaction.
4 Conclusions
We have proved by electrochemical measurements that in [Mo(η2-C60)2(CO)2(dbc-bipy)], which is the first ‘inorganic’ fullerene dimer symmetrically interposed by a metal fragment, the two cage subunits display a slight through-space electronic interaction, which even if Mo-mediated, is similar to that exhibited by the up-to-now known most interacting ‘organic’ bis-fullerenes.
Acknowledgements
P.Z. is indebted to Professor Kaluo Tang (Institute of Physical Chemistry, Peking University, China) for a sample of the bisfullerene under subject. The financial support from the University of Siena (PAR 2003) is gratefully acknowledged.



Vous devez vous connecter pour continuer.
S'authentifier