1 Introduction
The pioneering investigations into the chemical reactivity of [60]fullerene have provided a precedent towards the design and synthesis of novel and sophisticated architectures that may have applications in medicinal chemistry and the material sciences [1–4]. Further, with the increasing degree of complexity of fullerenyl derivatives comes the necessity for a larger range of reliable techniques for the unequivocal characterisation of such molecules. Such techniques should rely heavily on direct methods of characterisation, e.g. NMR spectroscopy, rather than the current, well used comparative techniques.
The concept of using the fullerene cage as a three-dimensional template in aspects of medicinal chemistry, nanotechnology and materials science is well established. The momentum for our initial investigations into fullerenyl chemistry was the notion that the complete chemical control of functionality on the surface of the C60 sphere would allow the precise placement of specific binding groups, permitting subsequent applications in medicinal chemistry with a large array of enzymatic binding pockets to be easily targeted. Clearly, this level of synthetic achievement is still a significant distance from reality, with not only current limitations on the available synthesic technology, but additional limitations in the unambiguous characterisation of these multifunctionalised fullerenyl derivatives.
With these principles in mind we embarked on a program to investigate the regioselective synthesis and characterisation of fullerenyl derivatives with multiple amino acid functionalities with a view to utilise such molecules as templates for molecules to be used in medicinal chemistry and nanotechnology. Our starting point was to try and synthesise fullerenyl α-amino acids, as such binding groups could have clear applications in biological systems – a number of such systems are illustrated in Fig. 1. The proline derivative 1 is already well known [5,6], whereas the more generic fullerenyl α-amino acids 2 and 3 had not been reported at the beginning of our studies.

Possible forms of fullerenyl α-amino acids.
2 Reaction of imino glycines
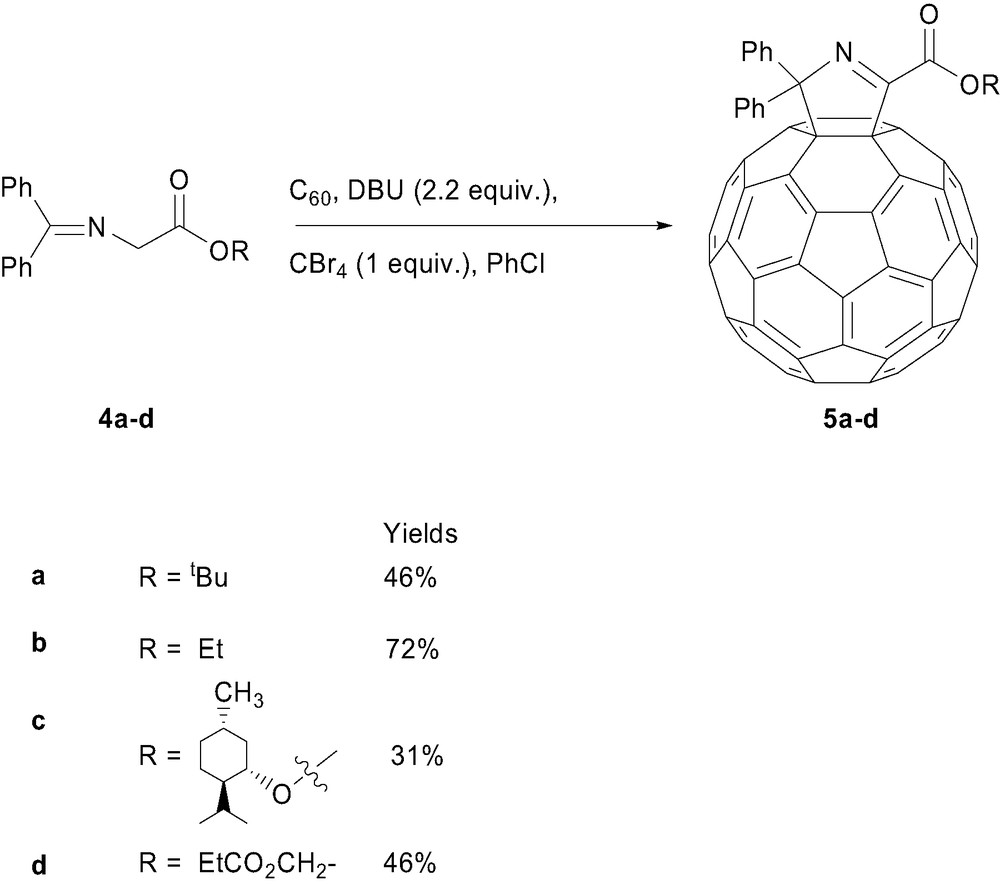
Our initial studies began with an examination of the addition of N-(diphenylmethylene)glycinate esters 4a–d to [60]fullerene under Bingel conditions (Scheme 1). We reported the synthesis of the methano[60]fullerenyl iminoesters 6 (Fig. 2) based upon extensive structural characterisation including the observation of a single sp3 fullerene resonance (between δ 82–83) in the 13C NMR spectra (4:6 CDCl3/CS2) of these compounds at 75 or 100 MHz, which implied to us that these molecules had Cs symmetry. Subsequent examination of the 13C NMR spectra (4:6 CDCl3/CS2) of these compounds at higher field (150 MHz) showed two sp3 fullerene resonances separated by 0.02–0.03 ppm (3–4.5 Hz) in this chemical shift region. When pure CDCl3 was employed as the solvent, these resonances were resolved by 16 Hz. This prompted us to re-examine our initial NMR and structural assignments and to perform INADEQUATE NMR experiments to unequivocally determine that the products of these reactions were [60]fullerenyldihydropyrroles 5a–d, as illustrated in Scheme 1 [9]. Further key evidence to this assignments were the HMBC experiments which indicated a strong correlation from the ortho protons of the phenyl substituents to a fullerenyl sp3 carbon atom (5, Fig. 2) and no correlation to any downfield resonance attributable to the imine group (as would be necessary in the presence of a three-membered ring structure, 6, Fig. 2). The assignment of the entire fullerene sphere was unequivocally determined by 2D-INADEQUATE and 13C NMR experiments using 10% 13C enriched fullerene. Fullerenyl resonances were distinguished from non-fullerenyl resonances by the presence of 13C–13C coupled satellites situated on either side of a central resonance peak. Assignment of the carbon sphere was achieved on the basis of one-bonded 13C–13C connectivities and examination of the carbon–carbon coupling (1JCC) values knowing typical values for C(sp2)–C(sp3) bonds (~48 Hz), the longer 5,6 ring-fused bonds (54–57 Hz) and the shorter 6,6 ring-fused bonds (65–71 Hz) [7–11].

The corresponding bisadducts, connected by the well known benzene dimethanol tether, reacted with C60 to give the expected [60]fullerenyldihydropyrroles bisadducts with the regiochemical outcome trans-4 and cis-3 in a 3.2:1 ratio for the ‘meta’ tether and trans-3 and trans-4 in a 3.7:1 ratio for the ‘para’ tether (Fig. 3). There was no bisadduct formed using the ‘ortho’ tether [11].
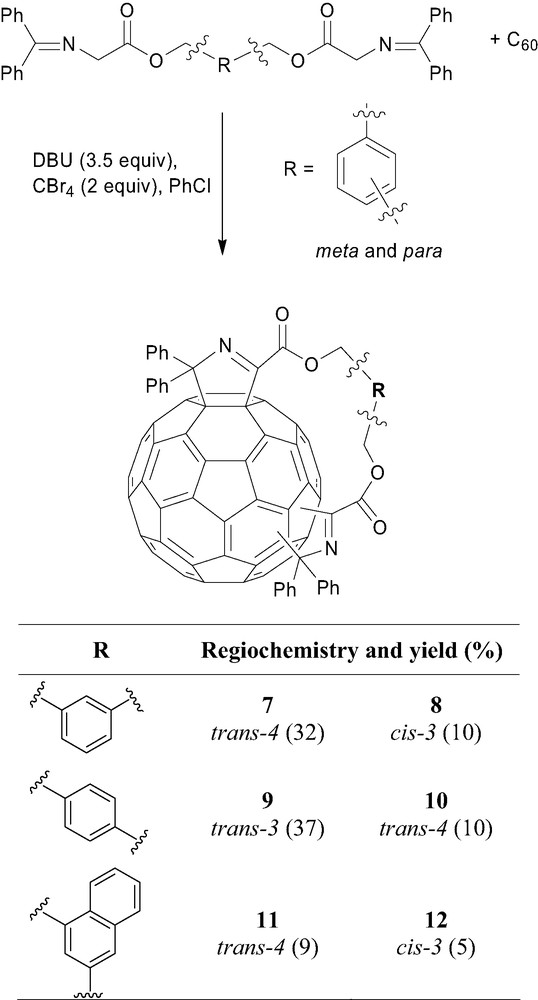
Synthesis of the bisadducts using iminoglycine addition chemistry.
The corresponding bis-malonate derivative, linked by the identical ‘meta’ tether, yielded exclusively the cis-2 bis-methano[60]fullerene [12]. Given the differences in regiochemistry using an identical tether, we decided to examine the regiochemical outcome of a mixed-tethered system utilising 1,3-benzene dimethanol to tether a N-(diphenylmethylene)glycinate and a malonate unit.
The reaction of 13 with [60]fullerene under Bingel conditions using CBr4 (2 equiv) and DBU (4 equiv) gave the e-edge-[60]fullerenylmethanodihydropyrrole 14 in a yield of 30%. This regiochemical outcome was the sole product regardless of the order of addition (initial reaction of the malonate moiety vs. iminoglycine addition) or whether the reaction was done in the presence of excess reagent or in a stepwise fashion. The regiochemical outcome was found to be independent of the order of addition of either the N-(diphenylmethylene)glycinate or the malonate moieties. The mixed-tethered system 13 gave a different regiochemical outcome (exclusively the e-edge-isomer) to the corresponding symmetrical tethered systems comprising a bis-malonate (cis-2 isomer) or a bis-iminoglycine (trans-4 and cis-3, 3:1). These differences indicate that these regiochemical outcomes are not dependent on the nature of the tether alone, but must incorporate additional factors including the mechanism of each reaction, the orientation of the tether based upon the first addend, and the electronic nature of the mono-substituted-fullerenyl changing the likely kinetic and thermodynamic outcomes of subsequent additions [13]. The first step in the cyclisation reactions of mono-adducts of these reactions to give bisadducts, was expected to involve the addition of an α-bromoenolate anion to a mono-substituted-[60]fullerene. This initial step may be reversible, however, the final step, attack of a fullerenyl anion on either the bromomalonate or the bromoiminoglycinate moiety would be expected to be irreversible and thus under kinetic control. Clearly both cyclisations favour formation of the e-edge-regioisomer in the second reaction step, independent of the nature of the first addend (Fig. 4).

Addition of the mixed malonate-iminoglycine tether to C60.
3 Ring-opening reactions
In an interesting reaction, reduction of the fullerenyl iminoesters 5a–d with sodium cyanoborohydride yielded the novel ring-opened 1,2-dihydro[60]fullerenylglycinates 18a–d in good yields [14]. The mechanism of this reaction is proposed to proceed via the intermediate 15 (Scheme 2), which undergoes ring-opening to give the more conjugated (stable) diphenylmethyleneimine, fullerenyl anion intermediate 17 (Scheme 3), rather than the less conjugated Ph2CHN=C(fullerenyl)(CO2R) imine, fullerenyl anion intermediate (not shown). Further reduction of the imine moiety of 17 and protonation gives the ring-opened 1,2-dihydro[60]fullerenylglycinates [9]. Extension of this reaction to the corresponding bisadducts 7–10 gives rise to the reduced ‘mono-adduct’ 19 (Fig. 5), presumably via a transient unstable dianion species and the driving force of addend removal arising from the transformation of sp3 to sp2 hybridisation [15]. Using identical reaction conditions the mixed-bisadduct 14 (Scheme 3) also yielded the corresponding ring-opened product in good yield [13]. Subsequent transesterification of 20 removed the tether and, in principal, indicated the ability to develop further chemistry around such systems. In general, one of the most interesting aspects of this unique ring-opening reaction is the possibilities for further chemistry, either utilising the protected amino or carboxylate functionalities, or the acidic fullerenyl hydrogen in a range of possible chemistries.
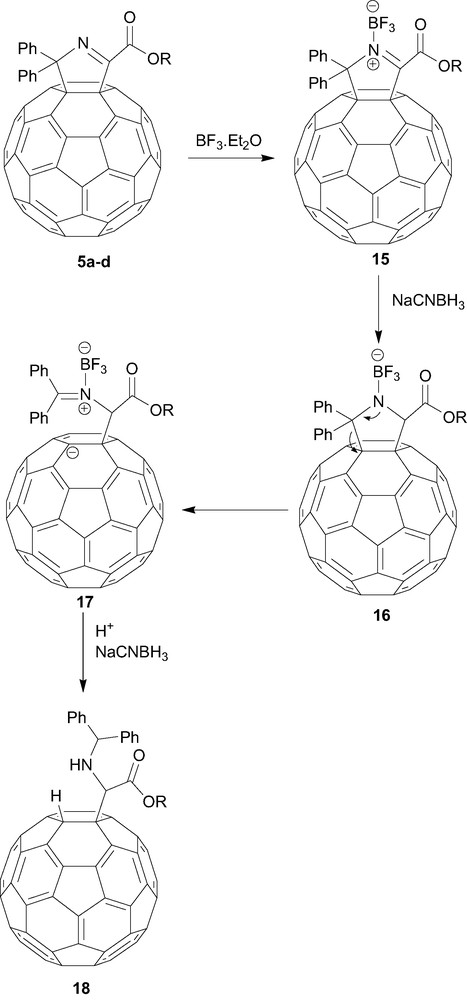
Reaction mechanism of the reductive ring-opening of fullerenylglycinates.
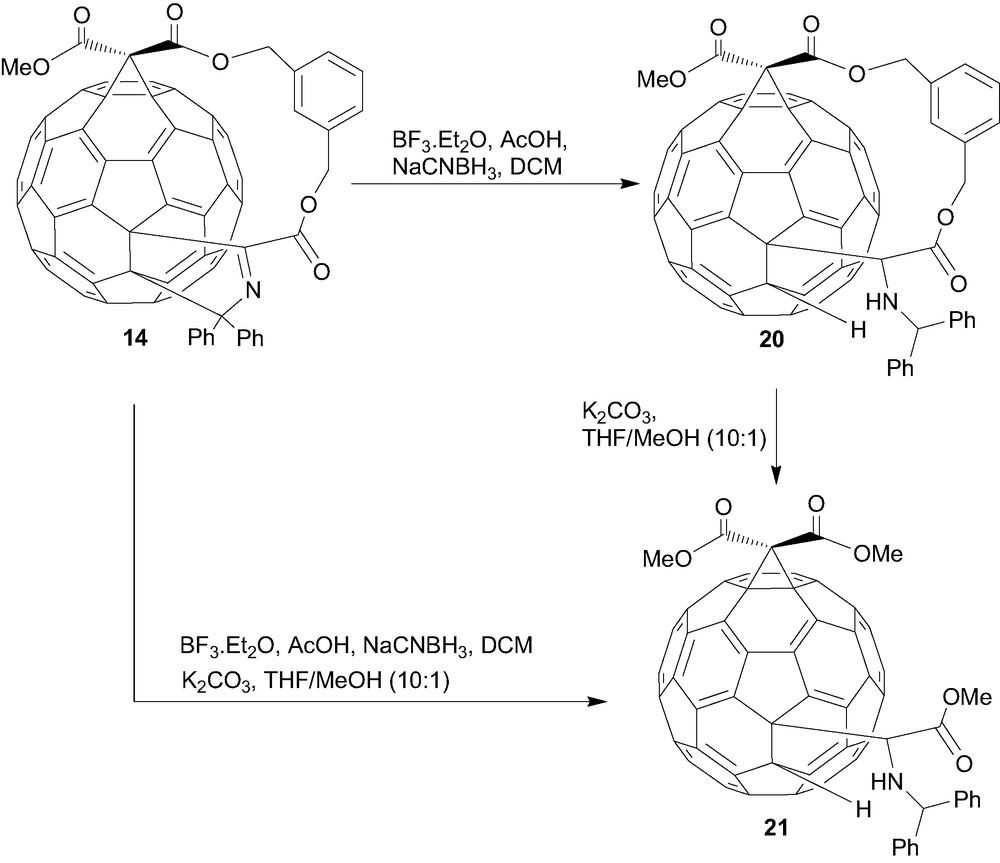
Reductive ring-opening of the mixed-tether bisadduct. Ring-opening followed by transesterification yields the identical outcome as the inverse order of reaction.
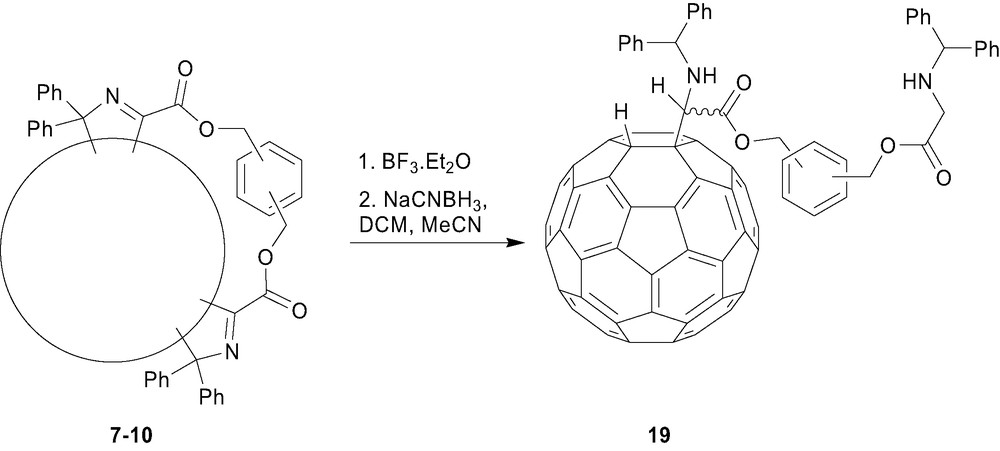
Ring-opening of the bisadducts 7–10.
4 NMR characterisation of C60 adducts
The unambiguous characterisation of our fullerenyl adducts by NMR spectroscopy is a key component of our studies. By assigning as completely as possible all carbon atoms in the molecule we ensure that there is no ambiguity in the structural assignment with no reliance on comparative techniques. The establishment of symmetry is a key initial step in this process with the identification of the number of full intensity and half-intensity peaks in the fullerenyl region of the 13C NMR spectra. A further key element is the unambiguous assignment of at least two different carbon atoms present on the surface of the sphere – these two atoms will be starting and finishing points for the sequence of stepwise correlations that will establish the relative positions of substituents present. As a straightforward example of this process, the mixed-tether bisadduct 14 was assigned using the INADEQUATE experiment illustrated in Fig. 6. The unambiguous assignment of the sp3 carbon atoms C1 and C3 was facilitated by previous well established experiments on the corresponding mono-adducts and represent the starting and finishing points for the series of correlations. The stepwise correlations are illustrated by dotted arrows and the ‘walk across the sphere’ from C1 to C3 unambiguously establishes the e-regiochemistry for this bisadduct. This particular example of the process is a relatively easy example as the two addends themselves are different (a cyclopropyl derivative and a five-membered pyrrolidine ring) and this gives rise to a greater dispersion of signals to assign to the sp2 fullerenyl carbon atoms. Further, due to the plane of symmetry that bisects the tether (Fig. 7), there are only 29 different fullerenyl carbon atoms to assign. These conditions significantly simplify the analysis and reduce the possibility of overlapping signals.

Assignment of the regiochemistry of the C60 mixed-tether adduct 14. The upper portion illustrates the entire spectrum and shows the starting and finishing points of C1 and C3. The lower portion is an expansion of the fullerenyl section of the spectrum.

Schlegel diagram of the mixed-tether adduct 14 showing the plane of symmetry – the 1,3-dimethylbenzene component of the tether is omitted for clarity.
5 Conclusions
Our initial studies on the addition of iminoglycines to C60 in attempts to produce protected amino acid derivatives led us to investigate other chemistry of significance to the fullerene area. This includes the synthesis of pyrrolidinofullerenyl adducts and subsequent reductive ring-opened derivatives. The chemistry is also applicable to multifunctionalised fullerenyls. The importance of full characterisation of adducts is also highlighted with the complete assignment of our structures by NMR spectroscopic techniques. We are currently extending this chemistry to include the synthesis of α-amino acid fullerenyl derivatives – as originally planned – and the investigation of higher-order fullerenyl adducts.
Acknowledgements
We thank the ARC Centre for Nanostructured Electromaterials for financial support and the University of Wollongong for partial support for a Ph.D. scholarship (B.C.H.).


