1 Introduction
Dextran is a naturally occurring non-toxic, biodegradable and microbial polysaccharide. Dextran and its derivatives are used as plasma expanders, blood substitutes, bone healing promoters, and also for the dermal and subcutaneous augmentations [1]. Furthermore, dextran is widely under investigation as a polymer carrier in novel drug delivery systems [2]. Dextran is usually produced from sugar by fermentation of extracellular dextransucrases, which contain different percentages of the branched α-1,3 linkages. However, since high molecular weight dextran could generate toxicity for the human body, so the fermented rough dextran cannot be used directly in a clinical context until it is hydrolyzed into that with low molecular weight [3]. Thus, studying the method of improving the purity of dextran with certain molecular weight is of technological importance, especially for clinical applications.
Certain organic solvents could influence the solubility and the crystallization kinetics (crystal nucleation and growth rate) of crystalline materials in solution, so they are widely used in extraction and refining of natural products. Ethanol grading sediment is a method of solvent crystallization, in which ethanol dissolved with moisture but unsolvable with solute makes solute dehydrated and crystals are formed while the solute concentration in water increases up to its saturated concentration [4–6]. Dextran can also be separated by this way from enzymic fluid.
However, the products are mixtures with different molecular weights, and quality problems induced by the disproportion of molecular weight were reported [7,8]. At the same time, the role of solvent–surface interactions in enhancing or inhibiting crystal growth is still not completely resolved [9].
In recent years, ultrafiltration, a well-established separation process in the biotechnology and fermentation industries [10], was used to separate polysaccharides [11–13]. The control of membrane fouling by the application of external DC (direct current) electric fields has been studied in several applications, and in some cases a clear enhancement has been achieved. This treatment technology has been found to be capable of reducing the formation of filter cake and increasing the flux of filtrate [14,15].
In this study, electric ultrafiltration-solvent crystallization (EUSC), which coupled electro-ultrafiltration (EU) with solvent crystallization (SC), is a new separation method proposed for the separation and purification of dextran. Our object is to separate dextran and control its molecular distribution at the same time, and prepare dextran with high purity.
2 Experiment
2.1 EUSC system
The five sections within the EUSC system were pump, charging basket, ultrafiltration, direct current source and crystallizer. Feed liquid could be concentrated and separated by the EU, as the organic solvent is added into the charging basket. The dynamic force of liquid flow is supplied by the pump. The experimental set-up used in this work is shown in Fig. 1.
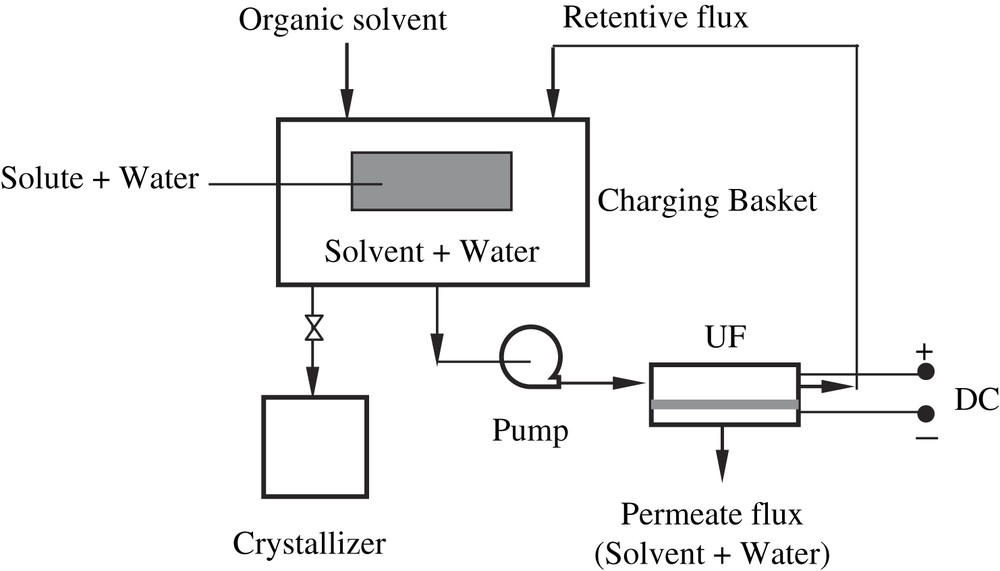
Schematic sketch of the EUSC process (total-reflux operation).
Within the EUSC system, the area of P10 membrane is 15 cm2 × 6 cm2 and the height of membrane cavity can be adjusted from 0.2 cm to 1.0 cm by replacing filling pieces. The membrane is made of polyethersulfone with 10-kDa molecular weight cut-off (MWCO) provided by Sunstar Membrane Technology Company, China. The pressure of EUSC system was provided by a WATSON323 constant-flow pump (Amersham Biosciences Company, US) with an entrance pressure of 0.10 MPa, and the DC electric field was provided by a WYI-24 electrical power unit (Golink Technology Company, China).
2.2 Materials
The commercial dextran D20, with an average molecular weight of about 20 kDa, was obtained from Shanghai Reagent Co. Ltd. (Shanghai, China). Ethanol was of reagent grade and was used without further purification.
The dextran solution was prepared by dissolving 30.00 g of dextran D20 in 300 mL of distilled water at room temperature, and agitated by an electric stirrer at 3000 rpm for 60 min in order to make dextran dissolve sufficiently. After that, dextran solutions were filtrated by a UF membrane with 30 kDa MWCO, and the permeated fluid was taken as specimen solution for the EUSC process.
2.3 Methods
Regurgitation operation was opted to increase the concentration of retention fluid quickly. The added quantity of ethanol was controlled at 5.0%, 10.0%, 15.0%, 20.0%, 25.0%, 30.0%, 40.0% and 60.0% (v/v) according to the capacity of the specimen solution. The proper addition quantity of ethanol was confirmed based on the permeate flux, and selected for the experiment of EUSC.
The mixed fluid of dextran and ethanol prepared with the proper addition quantity of ethanol was taken as the feed fluid for the EUSC process. Intensity of DC electrical field was exerted in the membrane separation with different voltages, such as 5 V, 10 V, 15 V, 20 V, 25 V and 30 V. The proper intensity of the DC electrical field was confirmed based on the permeate flux.
3 Results and discussion
3.1 The effect of ethanol concentration
It was reported that the presence of ethanol plays an important role during ultrafiltration of macromolecules [16]. Experiments were performed to determine the effects of different ethanol concentrations on the permeate flux of dextran. The permeate flux (J) was calculated by the time-consumption of getting 10 mL permeated fluid, and the average membrane flux (average J) based on the interval of getting 100 mL permeated fluid. Fig. 2 shows permeate flux as a function of solvent volume for different ethanol concentrations (0%–60% (v/v)). As expected, the membrane flux was obviously affected by the volume of added ethanol. As the dosage of ethanol increased, membrane permeate flux increased at the beginning, and then, decreased as the dosage of added ethanol was above 25% (v/v). It seems that proper dosage of ethanol could reduce the viscosity of the feed liquid, and expand the polarization time while gel formation was delayed. However, too much added ethanol could destroy the stability of dextran solution, and induce dextran crystallization on the face of membrane and block the membrane's holes.
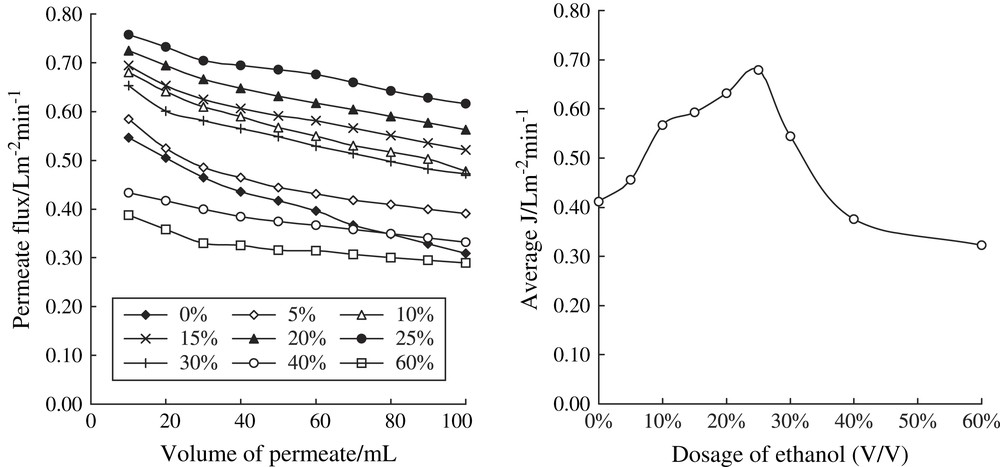
Permeate flux and average J of different dosages of ethanol.
3.2 The effect of electric field
The mixture fluid used for EUSC process was prepared by dextran and ethanol until ethanol dosage was about 25% (v/v). The intensity of DC electrical field was exerted in the membrane separation with different voltages between 5 V to 30 V. The effects of electric field on permeate flux are more clearly shown in Fig. 3. It was clear that DC electric field could distinctly improve the permeate flux of the Ultrafiltration-Solvent Crystallization (USC) process. The permeate flux increased with the increase in the DC voltage. What should be noted is that with increasing the voltage, the average permeate flux is increasing linearly at the beginning and then there is no obvious enhancement. It means that there is an optimal voltage of DC electric field while dealing with different consistencies of dextran solution. For 10% (w/v) dextran solution, the optimum voltage should be about 25 V. It seems that there was a critical voltage in EUSC processing, and it might be caused of the charges on the surface of dextran molecular. As the voltage increased, the electrophoresis transportation strengthened, thereby avoiding the exacerbation of the polarization concentration when the retentive flux is enriched. The critical electric voltage indicated that there was a definitive point, by which the diffusion and adsorption of the concentration polarization layer would be in equilibrium.

Permeate flux and average J of different electric fields.
3.3 HPLC chromatography
Dextran specimen D20 and separated dextran of EUSC (electric field 25 V, ethanol concentration 25%) were measured by the Waters HPLC system, which consists of a pump 515, a column heater CH-30, and a differential refractometer detector 996. Analysis was performed by TSK G2000 column, 5μm, 7.8 mm × 300 mm. Detector output was processed and manipulated by TL9900 software. The mobile phase used 0.7% Na2SO4 solution. Consistency of sample solution is 10 mg/mL, which was prepared with mobile phase. The conditions of chromatography include column temperature 35 °C, velocity of flow 1 mL/min and injection magnitude 20 μL.
According to the peak widths shown in Figs. 4 and 5, the molecular weight distribution of separated dextran was restrained into a small range by comparing with the original dextran specimen. It indicated that the separation and purification of rough dextran on EUSC was effective.
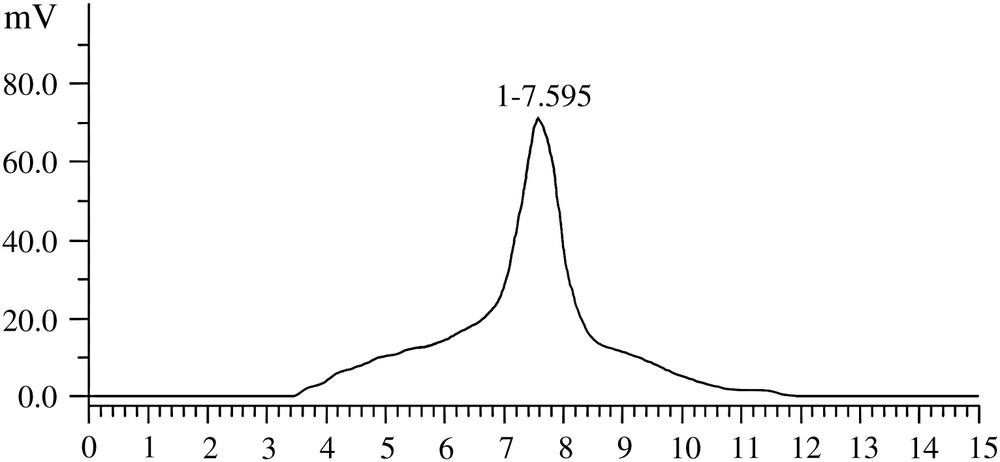
HPLC chromatogram of specimen dextran D20.

HPLC chromatogram of separated dextran by EUSC.
4 Conclusion
It was observed that the presence of ethanol plays an important role during ultrafiltration of clinical dextran, and an external DC electric field is effective to maintain the permeate flux of UF at a stable level. Moreover, the service life of EUSC system can be prolonged. As the viscosity of solution is not improved quickly, low molecular weight species can pass through the membrane, and higher molecular weight dextran could be concentrated and purified.
It was concluded that ultrafiltration of dextran is influenced by the formation of gel at higher dextran concentration, and electric filtration coupled with solvent crystallization can delay the concentration polarization time of the ultrafiltration process. However, excess ethanol could reduced the dissolvability of dextran in solvent, inducing dextran crystallization on the face of the membrane, thereby blocking the holes of the membrane.


