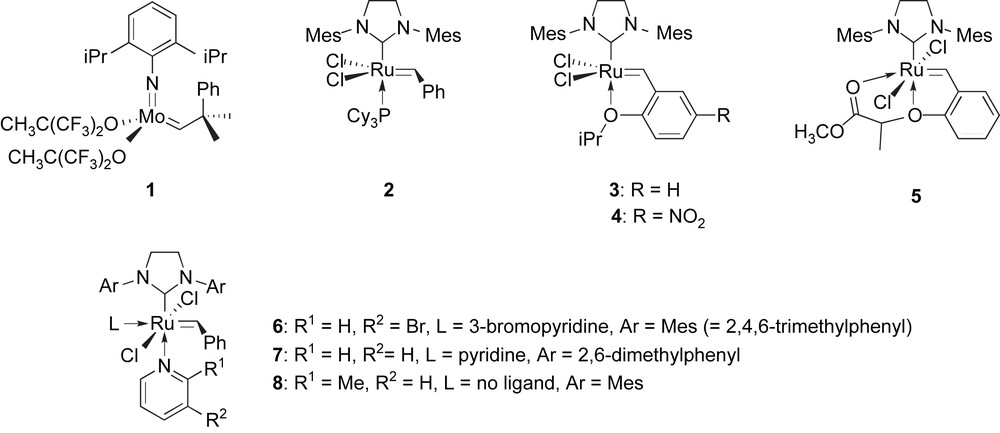
Olefin metathesis is now recognized as one of the most versatile and reliable methods for CC bond formation, and more specifically, the recent developments in ruthenium-catalyzed cross-coupling metathesis (CM) reactions have generated new synthetic opportunities [1]. However, some substrates and in particular acrylonitrile derivatives are still recognized as challenging cases due to their ability to deactivate or destroy the currently most routinely used catalysts. Acrylonitrile was early found inert in metal-catalyzed cross-metatheses and the first successful reaction involved the Schrock's molybdenum-based catalyst 1 to give, in sharp contrast with other CM reactions of conjugated electron-poor olefins, the kinetic (Z)-configured cross-coupling product as the major isomer [2]. The purity of acrylonitrile used in these reactions was later found to be of utmost importance [3]. Many improvements have been proposed over the past few years for the specific cross-metathesis of cyanide-containing olefins, and in particular with the now well-known Grubbs' ruthenium-based precatalysts 2 and 3 (Fig 1). For example, the addition of copper chloride as a phosphane scavenger combined with precatalyst 2 was reported [4]. Of great importance, Blechert and co-workers first described that the phosphine-free Hoveyda–Grubbs ruthenium carbene 3 could efficiently catalyze the CM of model olefins with acrylonitrile [5]. However, the issue of these CM stays highly dependant on the nature of the second partner. Indeed, some functionalized homoallyl alcohols [6], 3-silyloxyhexa-1,5-dienes [7] and 1,3-dienes [8] are reported to be poorly efficient partners in this reaction. Precatalysts 2 and 3 both allowed the cross-metathesis of allyl- and homoallyl-cyanide with moderate to good efficiency and the solution concentration was found crucial [9]. It should be noted that 1-cyano-1,3-dienes have recently been reported to undergo a moderately efficient CM with several olefins with precatalyst 2, although no reaction was observed with acrylonitrile itself as the second partner [10]. In a search for more active and fast-initiating precatalysts [11], complexes 4 [12] and 5 [13], analogues of the Hoveyda–Grubbs complex 3, were reported to catalyze efficiently the CM of acrylonitrile and remarkably also of methacrylonitrile with model olefins. Similarly, the mono- and bis-pyridine ruthenium complexes 6–8 are efficient promoters for the CM of acrylonitrile [14]. With other analogues of complex 3 bearing an asymmetrical N-heterocyclic carbene (NHC) ligand, a remarkable reversal of the E/Z selectivity was observed yielding predominantly the (E)-isomer, though with modest efficiency [15].
In recent years, microwave irradiation has been proposed as a complementary activation mode for olefin metathesis, and in many cases has resulted in a dramatic shortening of reaction times, and more importantly has allowed otherwise unproductive metathesis reactions [16]. In particular, the efficiency of the CM of β-ketoesters with acrylic derivatives was previously reported to be highly improved under microwave irradiation [17]. Following this study, we reported in an isolated example that microwave irradiation can indeed efficiently promote the cross-metathesis of acrylonitrile (2 equiv) with catalyst 3 in an unfavorable case [18]. The conditions have now been applied successfully to a number of challenging cases and, of significant importance in the context of eco-compatibility,1 with only 1 equiv of both olefinic partners.
For this study, a series of cyclic β-ketoesters and β-ketoamides 9 with a pendant olefin were prepared under eco-compatible conditions by microwave-assisted Wolff rearrangement of cyclic 2-diazo-1,3-diketones in the presence of the corresponding alcohol or amine [19a], except for 9f and 9i, j which were prepared by trans-esterification [19b] or trans-amidation [19c] of the corresponding methyl or ethyl β-ketoesters. All reactions were conducted with an equimolar amount of both olefinic partners in a vigorously stirred dichloromethane solution with 4 mol% of catalyst 3 added in two portions (3 then 1 mol%) in a sealed vessel heated at 100 °C [20] under microwave irradiation for 30 min.2 The results are summarized in Table 1. Under these conditions, we were delighted to observe that almost no loss of efficiency was observed in the CM of 9a with only one equivalent of acrylonitrile (entry 1 vs 2), and the E/Z ratio was not affected (ca. E/Z = 1:3 in all cases). Various other homoallyl β-ketoesters and β-ketoamides 9b–f were next engaged in CM reactions with 1 equiv of acrylonitrile with good efficiencies (entries 3–7). With substituted homoallyl olefins, a slight decrease of efficiency was logically observed (entries 4 and 5), and the slightly lower yield with 9f is attributed to a partial decarboxylation of 9f and/or 10f under the reaction conditions (entry 7). Next, the more challenging cases of allyl β-ketoesters and β-ketoamides 9g–j, which are prone to form an inert six-membered ring ruthenium chelate [21], were tested. As expected, in the allyl series the desired products were obtained with decreased but still productive efficiencies with only 1 equiv of acrylonitrile (entries 8–11). These very encouraging results prompted us to test the challenging cross-metathesis of 9a with methacrylonitrile (1 equiv), but in this case only traces [MS(ESI+) analysis] of the desired cross-coupling product were detected in the crude reaction mixture, which was essentially composed of the (E)-homodimer of 9a. The treatment of a CD2Cl2 solution of acrylonitrile with 3 (4 mol%) under microwave irradiation (100 °C for 30 min.) did not provide any detectable amount (NMR analysis) of the expected homodimer, confirming the type III character of acrylonitrile in the Grubbs categorization of olefins with catalyst 3 [22,23]. Although microwave irradiation clearly allows fast, clean and efficient cross-metathesis reactions of acrylonitrile with catalyst 3, it does not broaden its reactivity scope. The rigorous comparison of classical and microwave-assisted heating for olefin cross-metathesis has previously showed a beneficial effect of microwave irradiation [17,18]. We believe that an important contribution of microwaves in these reactions is the suppression of the wall effect (a thermal effect) which may result in a reduced rate of catalyst's decomposition and consequently a higher turn-over of the catalyst [16].
Microwave-assisted cross-metatheses with acrylonitrile (1 equiv).
| Entry | Substrate | Product | Yielda (%) |
| 1 | 85 | ||
| 2b | 89 | ||
| 3 | 90 | ||
| 4 | 60 | ||
| 5 | 51 | ||
| 6 | 79 | ||
| 7 | 68 | ||
| 8 | 24c | ||
| 9 | 52 | ||
| 10 | 38 | ||
| 11 | 30 |
a Based on isolated product after flash chromatography. All products were characterized by 1H and 13C NMR and MS ESI+ analyses.
b Reaction performed with 2 equiv of acrylonitrile. See reference [18].
c Estimated from the crude 1H and 13C NMR spectra due to instability.
In conclusion, microwave irradiation combined with precatalyst 3 allows the cross-metathesis of various olefins with only 1 equiv of acrylonitrile, a relatively low catalyst loading, and also a reduced consumption of energy when compared to classical heating. In the current era where the eco-compatibility of chemical processes is becoming an increasingly important parameter, we believe that microwave irradiation will soon become the first choice to perform olefin metathesis reactions.
Acknowledgements
We thank the French Research Ministry for a fellowship award to T.B., and the Université Paul Cézanne, the CNRS (UMR 6263) and the Conseil Général des Bouches-du-Rhônes for financial support.
1 “Eco-” is used here for both economical and ecological.
2 The procedure for 10a is representative: Microwave irradiations were performed with a CEM Discover 1–300 W in sealed tubes (10 mL) equipped with a Teflon-coated stirring bar under an argon atmosphere. To a solution of 9a (73.0 mg, 0.40 mmol) in dichloromethane (4 mL) were added acrylonitrile (26 μL, 0.40 mmol) and catalyst 3 (7.5 mg, 3 mol%). The reaction mixture was irradiated at 100 °C for 20 min and cooled down to room temperature. 2.5 mg (1 mol%) of catalyst 3 were added and the mixture was irradiated at 100 °C for an additional 10 min and cooled down to room temperature. The solvent and volatiles were evaporated under reduced pressure and the residue was purified by flash chromatography on silica gel to afford 70.5 mg of 10a (85%) as a brown oil.


