1 Introduction
The separation and removal of metal ions and neutral chemicals from water has frequently been addressed in membrane separation systems. Liquid membrane processes have become an attractive alternative to conventional solvent extraction for selective separation and concentration of compounds such as metals and acids from dilute aqueous solution because they combine in a single stage, an extraction and a stripping operation. In order to reduce the amounts of reactants and energy needed for separation and to decrease the environmental and economic impact of solvent extraction separations, several membrane-based separation techniques have been proposed in the past 30 years. Among these, supported liquid membranes (SLM) have been extensively studied since they offer high transport rates and good selectivity [1–11], and therefore are a very interesting option to overcome the solvent extraction downside (see [12] for a recent review on SLM-based separations). However, despite these advantages, it is commonly known that SLM are not widely used on an industrial scale nowadays, mainly due to their poor stability and subsequently short lifetime.
The carrier mediated transport of metal ions across liquid membranes is one of the promising options for the recovery of valuable metals from various waste streams. This is of great relevance in the nuclear industry in view of the stringent nuclear waste management regulations. In this context, a few attempts have been made for the recovery of radiologically toxic long-lived actinides and fission products viz. 241Am, 90Sr, 137Cs, employing extractants like octyl(phenyl)-N,N-diisobutyl carbamoyl methyl phosphine oxide (CMPO), dimethyl dibutyl tetradecyl-1,3-malonamide (DMDBTDMA) and crown ethers using membrane-based techniques [13–18].
Conventionally, the solvent extraction process has been used for the recovery of 233U from bulk thorium [19]. Furthermore, N,N-dialkyl aliphatic amides have received considerable attention as extractants for actinides from acidic media. Branched chain di(2-ethylhexyl) isobutyramide (D2EHIBA) has been synthesized and extensively evaluated as an alternate extractant to TBP for the separation of 233U from irradiated thorium [20–24]. In addition, carrier mediated transport offers several advantages over classical solvent extraction processes viz. minimisation of the loss of the carrier (the extractant) by entrainment, simultaneous extraction and stripping of the metal ions, high concentration factors, and very limited energy consumption.
A Polymer Inclusion Membrane (PIM) in some cases can incorporate only a base polymer and a suitable extractant while in other cases a plasticizer may also need to be added for obtaining a homogeneous and flexible membrane. PIM systems have been successfully designed for metal extraction using solvating-type extractants such as crown ethers, TOPO, TBP and β-diketones [25–38]. Several other kinds of carriers (alkyldithiophosphoric acids, alkylammonium salts and natural ionophores) have also been employed for this purpose [39–41].
In this work, we have synthesized a novel class of plasticized cellulose triacetate (CTA) membranes modified by carrier incorporation that are selectively permeable to uranium and molybdenum cations. The membrane CTA + NPOE + TOPO was characterised using chemical techniques as well as Fourier Transform Infrared (FTIR), X-ray diffraction and Scanning Electron Microscopy (SEM).
A comparative study of transport mechanism across such a plasticized CTA membrane called PIM and a SLM containing the same carrier in chloroform has been made.
2 Experimental part
2.1 Chemicals
Uranium (VI) nitrate and molybdenum (VI) nitrate, chloroform, CTA, and 2-nitrophenyloctyl ether (NPOE) were analytical grade reagents purchased from Fluka (Buchs, Switzerland). The carrier, trioctylphosphine oxyde (TOPO), was a product of Aldrich (Steinheim, Germany). All reagents were used as received without further purification. The aqueous phases were prepared by dissolving the different reagents in distilled water.
2.2 Membranes preparation
The CTA membranes were prepared according to the procedure reported by Sugiura et al. [29]. A chloroform solution (20 mL) of CTA (200 mg), the appropriate plasticizer NPOE (0.2 mL) and the above-listed TOPO in various quantities were placed in a 9.2 cm diameter flat bottom glass petri dish. The solvent was allowed to evaporate slowly overnight to obtain a polymer film [42,43] with a smooth looking surface. The obtained film was then carefully peeled out from the dish and placed in the transport cell with the side exposed to the air during evaporation facing the strip solution.
Polypropylene microporous membrane (SLM) of different characteristics: Celgard 2500 (porosity ɛ = 45%, pore size Φ = 0.04 μm, thickness d0 = 25 μm) (Hoechst Celanese Corporation, North Carolina) were used as supports. The immobilized liquid membrane was prepared by soaking the porous polymer in the carrier solution.
2.3 Analysis
The metal concentrations were determined by sampling at different time intervals aliquots of 0.5 mL each from the feed and strip solutions and analysed with UV visible spectrophotometer: Cintra 40 (GBC) at 652 nm. Mass flux J (mol.cm2.s−1) of the metal ions through the PIM transferred from the feed side of the membrane to the strip side was determined applying its definition: J = Δn/SΔt, where Δn represents the variation in mole number of metal ions in the receiving solution during the reference time Δt, and S is the membrane active surface. IR spectra were recorded on with Perkin-Elmer (Spectrum One) spectrophotometer. X-ray analyses were recorded on a BRUKER D8 diffractometer.
2.4 Transport
The cell used for transport experiments consisted of two compartments, made of teflon with a maximum filling volume of 50 mL, separated by the membrane. The feed compartment contains the metal solution at a concentration of 10−2 M of metal salt, the other compartment contains distilled water. Each compartment was provided with a vertical mechanical stirrer at stirring speed 800 rpm, which was previously determined as high enough to minimise the thickness of the boundary layer.
The experiments began when starting the stirring motors in the two compartments of the cell. The exposed membrane area was 9.61 cm2. All the experiments were performed in a thermostat at 25 °C. The experiment duration has been fixed to 7 days and 0.5 mL of solution has been taken up in regular time intervals (24 hours) from the strip compartment in order to determinate uranium and molybdenum ion flux through the membrane.
3 Results
3.1 Physical and chemical characteristics of cellulose triacetate membranes
In Table 1, some of the characteristics of the membrane made with the carriers have been listed in comparison with those of the reference CTA membrane. As the carrier molecules are hydrophobic, the location of carrier molecules at the surface of the CTA modified membranes should modify the contact angle which is a parameter indicative of the wetting character of a material (θ = 46° in the case of hydrophilic CTA membrane and θ = ∼86° in the case of hydrophobic CTA + NPOE + carrier membranes).
Chemical and physical characteristics of cellulose triacetate membranes.
| Membrane | Composition (weight of carrier) g | Thickness (μm) | Weight/Area (mg/cm2) | Water content (%) | Contact angle θ (°) |
| CTA | 0 | 10 | 4.88 | 32.6 | 46.4 |
| CTA + NPOE | 0 | 15 | 6.11 | 22.5 | 80.5 |
| CTA + NPOE + TOPO | 0.045 | 31 | 6.59 | 13.8 | 82.4 |
| CTA + NPOE + TOPO | 0.090 | 34 | 7.38 | 6.6 | 84.6 |
| CTA + NPOE + TOPO | 0.135 | 37 | 8.17 | 3.8 | 86.1 |
As shown in Table 1, we observed that inclusion of carriers into CTA membrane induced a remarkable increase of its thickness (10 to 37 μm). This increase roughly parallels that of carrier molecular weight.
Our results showed that TOPO inclusion in CTA membrane resulted in a heterogeneous and amorphous PIM whose physical properties, such as thickness, density and hydrophobicity, are modified.
3.2 FTIR
Table 2 collects the peak values of the reference CTA, CTA + NPOE and CTA + NPOE + TOPO membranes. The obtained results showed the absorption band located around 1754 cm−1, which is attributed to stretching vibrations of the carbonyl group. Bands at 1219 and 1055 cm−1 correspond to the stretching modes of C-O single bonds. Less intense bands at 2944 and 2880 cm−1 are attributed to C-H bonds and the wide band detected in the 3500–3100 cm−1 region is attributed to the O-H bonds stretching modes.
Peak values and the corresponding radicals in different membranes. m (TAC) = 0.2 g, v (NPOE) = 0.2 mL.
| Membrane | Peak value (cm−1) | Corresponding radical |
| CTA | 3450 | O – H |
| 2944 | C – H | |
| 1754 | C=O | |
| 1527 | COO− | |
| CTA + NPOE | 3480–3550 | O-H (TAC) |
| 2935 | C-H | |
| 1755 | C=O (TAC) | |
| 1603 | -NO2 (NPOE) | |
| 1532 | N=O (NPOE) | |
| 1497 | C=C (NPOE) | |
| 1246 | C-O-C asym | |
| 1054 | C-O-C sym | |
| 856 | C-N (NPOE) | |
| CTA + NPOE + TOPO | Same bonds and | |
| 1146 | P=O (TOPO) | |
| 1466 | -PCH3 (TOPO) |
The same table shows the bands at 1532 and 1497 cm−1 correspond respectively to the stretching modes of N=O bonds and C=C bonds of NPOE. The band at 1603 cm−1 is due to the stretching modes of – NO2 bonds of NPOE. The bands at 1466 and 1146 cm−1 correspond respectively to the stretching modes of –P–CH3 single bond and P=O bond of TOPO.
The obtained results showed that all the maximum values extracted from the spectrum of the CTA reference membrane, i.e. without plasticizer and carrier, are present in the modified membranes spectra in addition to those of the carrier molecules that also involve the same radicals.
3.3 Scanning Electron Microscopy (SEM)
A 5 KV scanning electron microscope (SEM) (Hitachi S4500) was used to elucidate membrane morphology. Membrane samples were prepared by freezing under liquid nitrogen (70 K) and rapid fracturing, resulting in a clean break fracture image to view the cross-section. The samples were mounted with conductive glue to metal stubs with the fractured edge up and then coated with gold by sputtering. These samples were then viewed in the SEM at around 1000 × magnification.
The morphologies of the CTA membrane (Fig. 1: view of surface) show that this membrane constituted by polymeric CTA presents a porous structure, the distribution of the pores is nearly uniform (porosity = 50%). On the other hand, the CTA + NPOE and CTA + NPOE + TOPO membranes present a dense structure where the pores of membrane have been filled by the NPOE and TOPO molecules yielding to a thicker and less porous membrane.
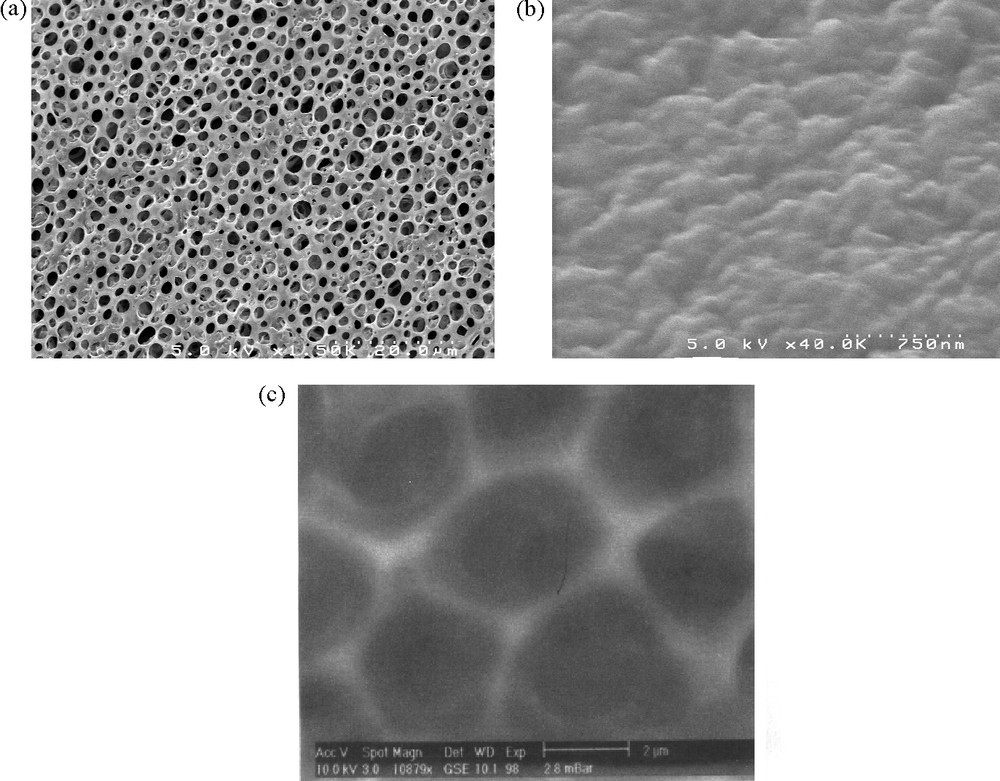
Scanning electronic microscopy of various membranes: (a): CTA membrane; (b): (CTA + NPOE) membrane; (c): (CTA + NPOE + TOPO) membrane.
Interestingly, surface of the CTA + NPOE + TOPO membrane presents a quite organised hexagonal network.
3.4 X-ray diffraction
Figs. 2 and 3 show the X-ray curves for CTA + NPOE membrane and CTA + NPOE + TOPO membrane. Based on these figures, we can observe the following.
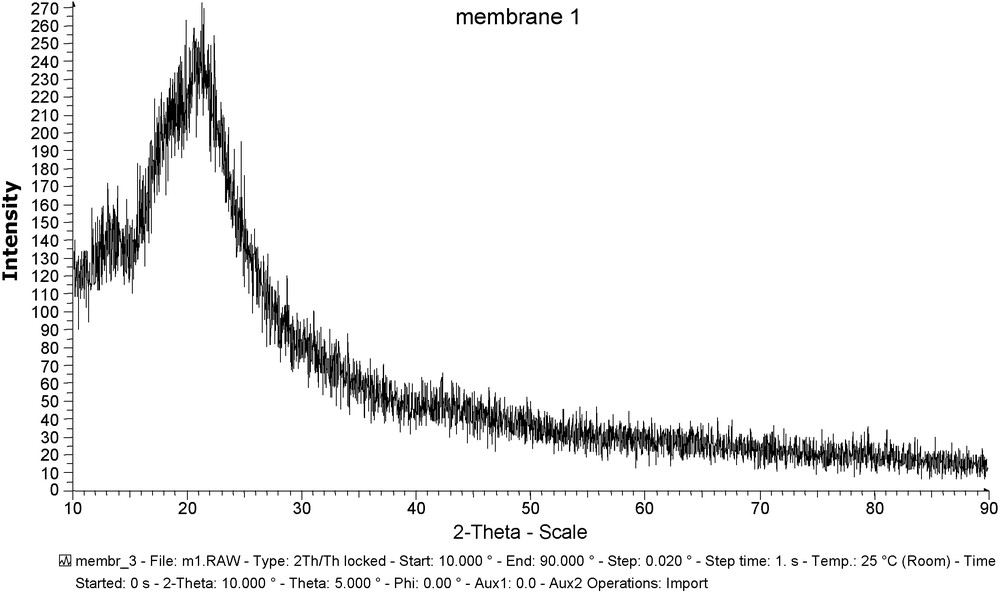
X-ray curve for CTA + NPOE membrane.
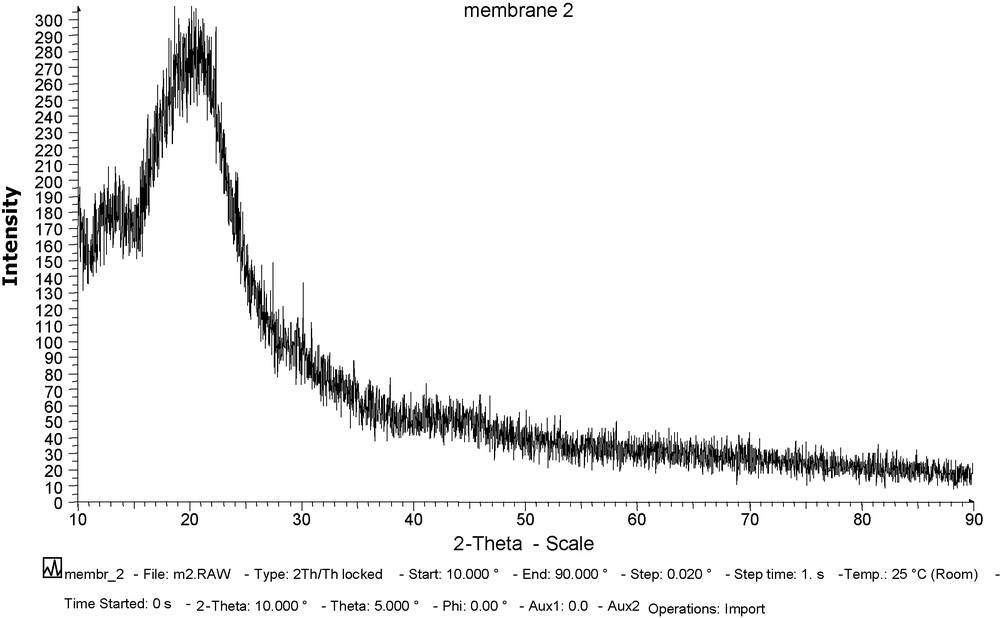
X-ray curve for CTA + NPOE + TOPO membrane.
The CTA + NPOE membrane presents a single maximum located at approximately 20° found in all polymers and corresponds to the Van deer Waals [44,45] halo. Thus, this material presents basically amorphous characteristics. The system constituted by the mixture of CTA + NPOE + TOPO do not give any diffraction. It can be due to the absence of crystallization within the membrane.
There are two possible mechanisms existing in the literature which can explain the diffusion of ions from the feed to the strip compartment:
- • membrane with amorphous structure: the mechanism is based on the simple diffusion through the SLM;
- • membrane with crystalline structure: the mechanism is based on the successive complexation–decomplexation from the feed to the strip compartment by jumping electron between the donor oxygen and phosphorus of TOPO and the metal as acceptor.
With the latter mechanism, the transporter molecules act as stepping stones, and the solute moves through the membrane by jumping from one fixed site to another. The theory of fixed-site jumping was described by Cussler et al. [46] and Noble [47].
Therefore, this result should be attributed to the amorphous state of the structure which permits us to eliminate the mechanism of transfer of the ions by successive jumps between carrier complexing sites in a 3D assembled state.
3.5 Effect of the quantity of carrier fixed in the membrane on transport efficiency
TOPO is widely used for the separation of 233U from irradiated toxic metals, particularly molybdenum and thorium. It is used as a solvent extractant of rare earth metals from ores. This is an organic phosphor compound, which forms stable, hydrophobic complexes with metals such as uranium and molybdenum. TOPO is soluble in chloroform and a solution TOPO/CHCl3 is miscible with NPOE.
The effect of the quantity of carrier fixed in the membrane was studied. Fig. 4 shows that uranium concentration in a strip compartment increases with the TOPO quantity and reaches a maximum value with a membrane containing 0.09 g of TOPO. Beyond this value, the ion concentration decreases using a quantity of 0.135 g of carrier. A quantity of 0.09 g of TOPO fixed in the membrane represents a saturation of the membrane with the metallic ion complexes, which can be explained by the equality of the chemical potential values in the feed and strip compartments. It is also due to the solubility limit of TOPO in the 2-nitrophenyloctyl ether (NPOE).
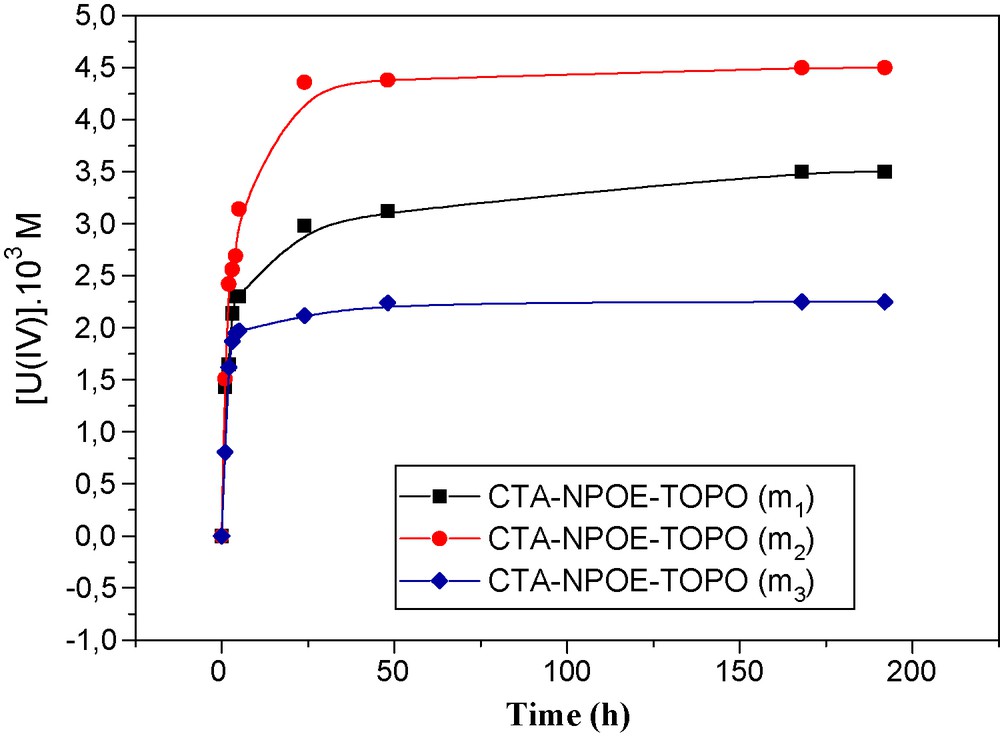
Variation of the concentration of uranium in the strip phase vs. time changing the quantity of TOPO in the membrane composition. m1 = 0.045 g; m2 = 0.09 g; m3 = 0.135 g; [Metal] initial = 10−2 M.
3.6 Uranium (VI) and molybdenum (VI) transport experiments
Fig. 5 represents the evolution of uranium (VI) and molybdenum (VI) ions concentration in the feed and strip compartments as a function of time using (CTA + NPOE + TOPO) membrane (m2 = 0.09 g) and the initial metal concentration in the feed compartment: [Metal] = 10−2 M.
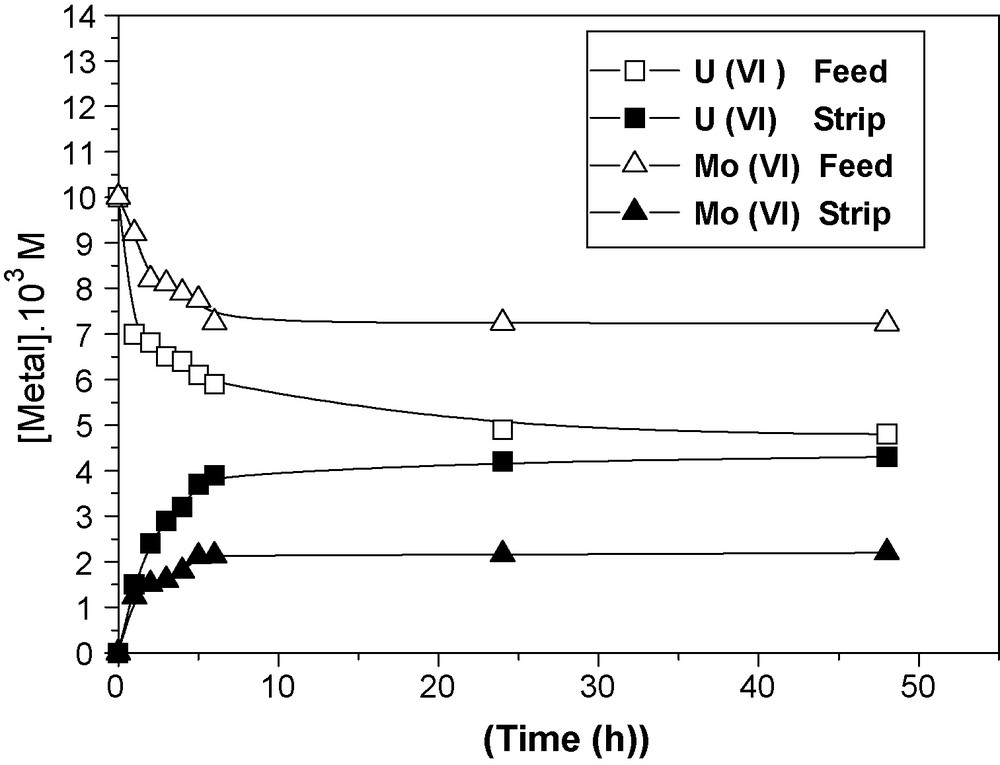
Evolution of uranium and molybdenum ions concentration in the feed and strip compartments as a function of time. Membrane: CTA + NPOE + TOPO (m2 = 0.09 g) [Metal] = 10−2 M.
The increase of uranium and molybdenum ion concentration with time in the strip compartment for the CTA + NPOE + TOPO membrane indicates that the transport is as expected facilitated by the introduction of the carrier (transport percentages of metal ions without carrier ∼5%).
A comparative study of the transport across a PIM and a SLM containing the same carrier has shown that uranium transport efficiency was increased using PIM instead of SLM. The transport efficiency was increased by a factor as high than five with TOPO carrier when PIM (flux ∼21.5 × 10−10 mol.cm−2.s−1) were used in comparison to SLM (flux = 3.75 × 10−10 mol.cm−2.s−1).
3.7 Membranes stability
Repeated transport tests of the uranium ion were carried out for characterising the stability of the PIM. Feed and strip phases were renewed every 24 h without changing the membrane. For each test, the average flux during 24 h, before renewing solutions was measured and plotted as shown in Fig. 6. The stability study of the PIM shows that, contrary to the case of the SLM, the flux of transport remains constant after 2 weeks.
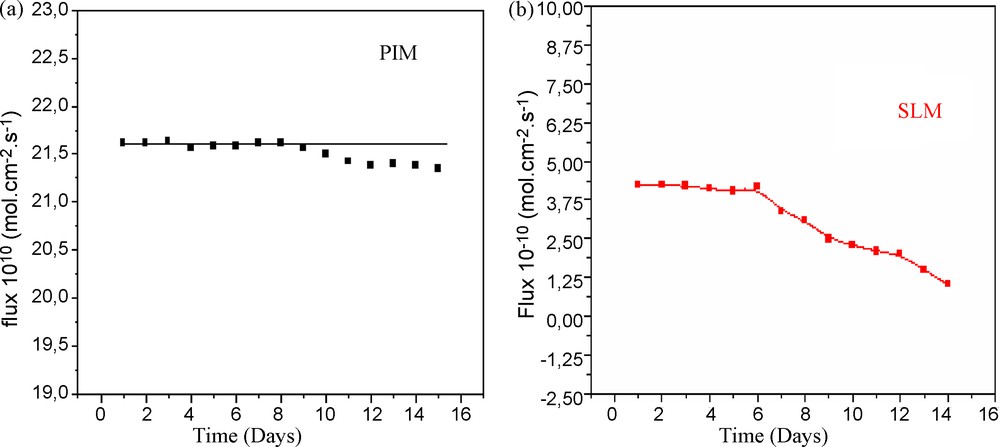
Evolution of the transport mass flux vs. number of runs. a: PIM: CTA + NPOE + TOPO; b: SLM: Celgard 2500, [U (VI)] = 10−2 M.
The decay of flux in SLM can be attributed to specific interactions between the organic solvent, carrier and the support: decrease of membrane porosity due to pores plugging. These effects were completely avoided in the case of PIMs. However, the stability is significantly improved with PIM.
Due to the mixing of the carrier with the CTA polymer in PIM, the release of the carrier from membrane phase to the aqueous phases which occurs when SLM membrane are used is avoided, since in PIM the carrier remains fixed within the membrane matrix because of absence of actual liquid solvent.
4 Conclusion
A CTA membrane containing TOPO as a carrier and 2-nitrophenyloctyl ether (NPOE) as a plasticizer has been synthesized. These CTA + NPOE + TOPO membranes were characterised using chemical techniques as well as FTIR, X-ray diffraction and SEM. Transport studies using PIM containing TOPO in 2-nitrophenyloctyl ether (NPOE) has shown that the uranium and molybdenum ion transport efficiency was increased using PIM instead of SLM. The transport efficiency was increased by a factor as high than five with TOPO carrier when PIM (flux ∼21.5 × 10−10 mol.cm−2.s−1) were used in comparison to SLM (flux = 3.75 × 10−10 mol.cm−2.s−1).
The flux of transport PIM showed higher operational stability than SLM and remain constant after 2 weeks. The inclusion of a selective TOPO in the matrix of a polymer CTA gives rise to stable membranes able to transport ions and to work for a long time. This approach opens large perspectives for utilising the TOPO carrier mediated transport technique in the treatment of hydrometallurgical solutions.
Further efforts will be directed to the determination of the nature of interactions between polymer and carrier by use of other materials and analysis as well.


