1 Introduction
Ionic liquids (ILs), the molten salts with melting points at or below ambient temperature, have attracted intense focus owing to their remarkable chemical and physical properties such as high thermal stability, [1–3] negligible vapor pressure, [4] high ionic conductivity [5]. They have been used as alternatives to volatile organic solvents for organic synthesis in homogeneous as well as biphasic processes [6–8].
Initially, the focus of research for ionic liquids was on their role as alternative solvents in organic synthesis; however, after synthesis of an ionic liquid derived from the antifungal drug miconazole a new concept of “task specific ionic liquids” (TSILs) was introduced [9,10]. There is vast scope of chemical structural modifications that can be introduced either in the anion, the cationic core, or substituent on the anion or cation in an ionic liquid, leading to ionic liquid with different properties. Functionalized ionic liquids (FILs) are prepared by introducing a specific functionality covalently tethered to the cation, or the anion, or a zwitterionic form of the salt [11–13]. The enduring popularity of these TSIL or FILs lies in the fact that both the cationic and anionic components can be varied and the ionic liquid can be tailored to a specific application. Moreover, the incorporation of functional groups can impart a particular capability to the ionic liquids, enhancing catalytic stability and reducing catalyst leaching [14–17].
Recently, understanding biological activity and toxicity of ionic liquids has been of great interest due to their increasing applications. They have been shown to have significant toxic effects on different bacteria and fungi. 1-Alkylquinolinium bromides have been studied for their antimicrobial and antibiofilm activities [18]. Pyridinium, imidazolium and quaternary ammonium salts have been found to be toxic for variety of bacteria and fungi [19]. It has been observed that toxicity increases with the length of n-alkyl substituent in the methylimidazolium cation whereas anion has minimal effect [20,21].
Schiff bases constitute an important class of organic compounds because of their biological, analytical and pharmaceutical applications [22]. They possess excellent characteristics, structural similarities with natural biological substances. They are well known for broad spectrum biological activities such as antiviral, [23] anticancer, [24] antifungal, [25] antibacterial [26] agents. The microbiocidal activities associated with them is due to the presence of the toxophoric C = N linkage in them. Relatively simple preparation procedures and the synthetic flexibility enable design of compounds with desired structural properties. It is expected that synthesis of ionic liquid tagged Schiff bases can have synergetic effects on their microbial inhibition. With this aim and in continuation of our interest in ionic liquids, [27–31] in this article we report synthesis of novel ionic liquid tagged Schiff bases and their antibacterial and antifungal activities.
2 Results and discussion
In most functionalized ionic liquids, quaternization of ring nitrogen by alkyl halide to produce 3-alkylimidazolium salt is done after attaching the functional group to imidazole at the N-1 position. These reactions are generally performed in a narrow region of temperature and solvent parameters due to competing side reaction of the alkylating reagent with the incorporated functional group. We have designed a novel approach for the synthesis of functionalized ionic liquids where this alkylation of functional group can be avoided (Scheme 1).
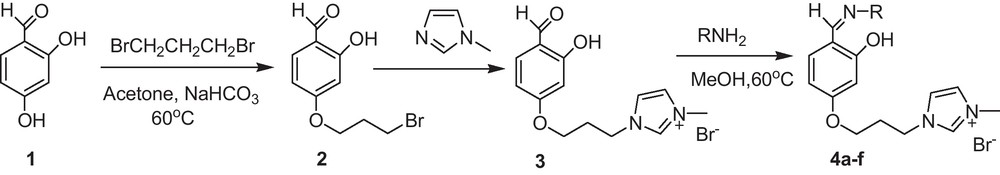
Synthesis of ionic liquid tagged Schiff bases.
The first step of the synthetic route involved the synthesis of 4-(3-bromopropoxy)-2-hydroxybenzaldehyde (2) by selective O-alkylation of 2,4-dihydroxybenzaldehyde (1). The reaction gives a mixture of 4-(3-bromopropoxy)-2-hydroxybenzaldehyde (2) and 4,4′-(propane-1,3-diylbis(oxy))bis(2-hydroxybenzaldehyde). Formation of dimeric 4,4′-(propane-1,3-diylbis(oxy))bis(2-hydroxybenzaldehyde) was minimized by dilution method. Usage of mild reaction conditions and presence of hydrogen bonding resulted in O-alkylation selectively at the para-positon. The 1H NMR spectrum of the compound showed singlet at δ 11.02 for OH, singlet at δ 10.02 for aldehydic proton, triplet at δ 3.66 for CH2Br, triplet at δ 4.10 for OCH2, multiplet at δ 2.28 for CH2 along with aromatic protons at δ 6.49-7.64. The ESI-MS spectrum of 2 displayed a peak at m/z 259.0 and 261.1 for [M]+ and [M + 2]+ ions, respectively. In the IR spectra a peak at 1624 cm−1 was observed for aldehydic C = O stretching and a sharper peak at 3080 cm−1 for OH stretching. There was no significant shift in the C = O stretching frequency that also indicates that intramolecular hydrogen bonding of ortho-hydroxy group with carbonyl group is not disturbed and alkylation has occurred selectively at the para-hydroxy group. In addition to this sharper peak at 3080 cm−1 for OH stretching also indicates alkylation at para-hydroxy group. In the subsequent step, 1-methylimidazole was reacted with 2 to form ionic liquid tagged aldehyde (3). The ESI-MS spectrum of 3 displayed a peak at m/z 261.2 for [M + H–Br]+ ion. The IH NMR spectrum showed an additional peak at δ 3.86 ppm for N-CH3 and three new peaks at δ 9.25, 7.84 and 7.74 ppm for imidazolium ring protons in addition to other protons. In the third step, 3 was reacted with different aromatic amines to yield ionic liquid tagged Schiff base (4). The yields of 4 were moderate and the nature of various substituents on the aromatic amines had no obvious effect on the yields. The details of yield and structure for different ionic liquid tagged Schiff bases are given in Table 1. Compound 4a exhibited absorption bands at 1620 cm−1 (C = N str.), 3100 cm−1 (H–C(=N) str.) and 3415 cm−1 (OH str.) in the IR spectrum. In 1H NMR azomethine proton appeared at δ 8.79 and hydroxyl proton at δ 13.77 along with other protons of the molecule. A peak appeared at δ 163.6 for azomethine carbon along with other carbons of the molecule in 13C NMR spectra. Similarly, spectroscopic data for all other compounds have been found to be consistent with the structure.
Synthesis of ionic liquid tagged Schiff bases 4a-f.
| Sr. No. | R | Structure of 3 | Yielda (%) | |
| 1 | C6H5 | 4a | 68 | |
| 2 | 4-BrC6H4 | 4b | 60 | |
| 3 | 4-ClC6H4 | 4c | 59 | |
| 4 | 4-FC6H4 | 4d | 64 | |
| 5 | 4-CH3OC6H4 | 4e | 68 | |
| 6 | C10H7 | 4f | 50 |
a Isolated yield.
Bactericidal assay of all the synthesized compounds (4a-f) was performed against common food pathogenic bacteria to evaluate their usability as efficient alternative to well-established chemotherapeutic agents. The zone of inhibition and minimum inhibitory concentration (MIC) values were measured to determine the antibacterial efficiency of the tested compounds. Gram positive and Gram negative bacterial strains were selected on the basis of their clinical importance.
The compounds (4a-f) exhibited moderate to good antibacterial activity against Gram positive bacteria (Table 2). This may be due to the interactions between peptidoglycan component of Gram positive cells and compounds which leads to disruption of cell wall resulting in cell death. Out of all the tested compounds, only 4a showed inhibition of both Gram positive and Gram negative bacteria, whereas 4d did not show any inhibition for both bacterial groups. Except 4a, all the compounds were found to be insensitive against the tested gram negative bacterial strains.
Zone of inhibition and minimum inhibitory concentration (MIC) values of compounds against Gram positive and Gram negative bacteria.
| Compound | Gram positive | Gram negative | ||||||||||
| B. cereus | S. aureus | P. putida | E. coli | K. pneumoniae | S. typhimurium | |||||||
| Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | |
| 4a | 11 | 128 | 13 | 64 | 13 | 64 | 11 | 128 | 12 | 64 | 11 | 128 |
| 4b | 10 | > 128 | 11 | 128 | 11 | 128 | * | > 128 | * | > 128 | * | > 128 |
| 4c | 10 | 128 | 11 | 128 | 10 | > 128 | * | >128 | * | > 128 | * | > 128 |
| 4d | * | > 128 | * | > 128 | * | > 128 | * | > 128 | * | > 128 | * | > 128 |
| 4e | 10 | > 128 | 12 | 64 | 10 | > 128 | * | > 128 | * | > 128 | * | > 128 |
| 4f | 11 | > 128 | 11 | 128 | 10 | > 128 | * | > 128 | * | > 128 | * | > 128 |
All the synthesized compounds (4a-f) were screened for in vitro antifungal activity. Table 3 shows zone of inhibition and MIC values for the tested pathogenic fungal species. Similar to the antibacterial activity, compound 4a was found to exhibit broad spectrum antifungal activity against all the tested fungi. The highest MIC value was observed with 4f followed by 4a and 4c against A. niger which was found to be the most sensitive fungal strain. Further studies are required to characterize the mode of action of these compounds against microbes.
Zone of inhibition values of compounds against various tested fungi.
| Compound | A. flavus | A. niger | F. oxysporum | C. acutatum | ||||
| Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | Zone of inhibition (mm) | MIC (μg mL−1) | |
| 4a | 15 | 64 | 16 | 64 | 15 | 64 | 15 | 64 |
| 4b | 14 | 128 | 13 | 128 | 13 | > 128 | 14 | 128 |
| 4c | 12 | > 128 | 16 | 64 | 15 | 64 | 14 | 128 |
| 4d | 13 | 128 | 13 | > 128 | 14 | 128 | 13 | > 128 |
| 4e | 13 | > 128 | 14 | 128 | 14 | 128 | 13 | > 128 |
| 4f | 14 | 128 | 18 | 32 | 14 | 128 | 15 | 64 |
3 Conclusions
In conclusion, a new approach for the synthesis of imidazolium ionic liquid tagged Schiff bases has been developed. Synthesis of ionic liquid tagged Schiff bases is achieved in three steps from 2,4-dihydroxybenzaldehyde by selective alkylation with 1,3-dibromopropane, followed by reaction with 1-methylimidazole and Schiff base formation with aromatic amines. The resulting compounds have been tested for antibacterial and antifungal activities. The present series of compounds exhibited significant antimicrobial activity against both bacteria and fungi which opens the avenue to utilize the compounds as broad spectrum chemotherapeutic agents. Further studies on the synthesis of a library of ionic liquid tagged Schiff bases and evaluation of their antimicrobial activity are in progress in our laboratory.
4 Experimental
4.1 General
All the solvents and reagents were purchased from Sigma-Aldrich, India and Spectrochem Pvt. Ltd., India and were used without further purification. Melting points were determined in open capillary tubes on a MPA120-Automated Melting Point apparatus and are uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker Heaven 11400 (400 MHz) and Varian (500 MHz) spectrometers using CDCl3 and DMSO-d6 as solvents and the chemical shifts were expressed in ppm. The bacterial cultures used were Bacillus cereus (MTCC 430), Staphylococcus aureus (MTCC 96), Pseudomonas putida (MTCC 102), Escherichia coli (MTCC 1652), Klebsiella pneumoniae (MTCC 432) and Salmonella typhimurium (MTCC 98). Aspergillus niger (MTCC 282), Aspergillus flavus (MTCC 6674), Fusarium oxysporum (MTCC 6045) and Colletotrichum acutatum (MTCC 2247) were used for antifungal studies. The axenic cultures of all strains were procured from MTCC, IMTECH, India and revived as per suggested guidelines using standard microbiological methods.
4.2 Synthesis of ionic liquid tagged aldehyde (3)
In the first step, reaction of 1,3-dibromopropane (8.0 mmol), 2,4-dihydroxybenzaldehyde (6.0 mmol) and sodium bicarbonate (6.0 mmol) in acetone (50 mL) at 60 °C for 60 hours gave mixture of 4-(3-bromopropoxy)-2-hydroxybenzaldehyde (2) and 4,4′-(propane-1,3-diylbis(oxy))bis(2-hydroxybenzaldehyde). Column chromatography over silica gel gave pure 2 in 65% yield. Reaction of 1-methylimidazole (3.5 mmol) with 2 (3.5 mol) at 80 °C for 48 hours gave a viscous liquid which was washed with diethyl ether-ethyl acetate mixture to give ionic liquid tagged aldehyde 3 in almost quantitative yield.
4.3 General procedure for synthesis of ionic liquid supported Schiff bases (4a-f)
A mixture containing ionic liquid tagged aldehyde (3.0 mmol) and amine (4.0 mmol) in ethanol was refluxed for 4 hours. On completion of the reaction, ethanol (30 mL) was added to the reaction mixture; the solid product formed was filtered off and washed with cold ethanol. The crude product was purified by recrystallization from ethanol/ethyl acetate (3: 1 v/v). All the compounds were characterized by ESI MS and 1H NMR and 13CNMR spectroscopic data.
Physical and spectral data for 4a-f:
4a: Yield 849 mg (68%); mp 81- 83 °C; IR cm−11620, 3416; 1H NMR (400 MHz, DMSO-d6) δ 13.77 (s, 1H), 9.48 (s, 1H), 8.79 (s, 1H), 8.20 (t, J = 1.8, 1H), 7.90 (t, J = 1.8 Hz, 1H), 7.79–7.78 (m, 1H), 7.69 (s, 1H), 7.57–7.48 (m, 3H), 7.29 (d, J = 7.2 Hz, 1H), 6.58–6.47 (m, 2H), 4.50 (t, J = 6.5 Hz, 2H), 4.15 (t, J = 5.1 Hz, 2H), 3.97 (s, 3H), 2.50–2.31 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.6, 162.9, 148.2, 148.7, 137.3, 134.7, 129.9, 129.3, 126.9, 124.0, 122.9, 121.6, 116.4, 114.6, 113.6, 107.5, 101.8, 65.4, 46.8, 36.2, 31.2, 29.4; ESI-MS (m/z): 336.3 [M - Br]+.
4b: Yield 891 mg (60%); mp 180-184 °C; IR (cm−1) 1612, 3425; 1H NMR (400 MHz, DMSO-d6) δ 13.42 (s, 1H), 9.35 (s, 1H), 8.77 (s, 1H), 7.80 (t, J = 1.8 Hz, 1H), 7.70 (t, J = 1.7 Hz, 1H), 7.55 (td, J = 8.7, 2.04 Hz, 2H), 7.46 (d, J = 8.5 Hz, 1H), 7.27 (td, J = 8.7, 2.04 Hz, 2H), 6.49–6.45 (m, 2H), 4.45 (t, J = 6.9 Hz, 2H), 4.12 (t, J = 5.8 Hz, 2H), 3.93 (s, 3H), 2.40–2.34 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.7, 163.4, 163.1, 147.7, 137.2, 134.7, 132.7, 124.1, 123.8, 122.9, 119.4, 113.6, 107.7, 101.8, 65.4, 46.8, 36.2, 31.2, 29.3; ESI-MS (m/z): 414.1 [M - Br]+, 416.1 [M + 2 - Br]+.
4c: Yield 797 mg (59%); mp 150-155 °C; IR (cm−1) 1612, 3325; 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 9.33 (s, 1H), 8.74 (s, 1H), 7.77 (t, J = 1.8 Hz, 1H), 7.67 (t, J = 1.7 Hz, 1H), 7.44 (d, J =8.2 2H), 7.41 (td, J = 8.8, 2.08 Hz, 2H), 7.31 (td, J = 8.8, 2.16 Hz, 2H), 6.50–6.42 (m, 2H), 4.44 (t, J = 6.9 Hz, 1H), 4.12 (t, J = 5.8 Hz, 1H), 3.93 (s, 3H), 2.40–2.34 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.6, 163.3, 163.1, 147.3, 137.3, 134.7, 131.1, 129.8, 128.9, 124.1, 123.5, 122.9, 115.9, 113.6, 107.6, 101.8, 65.4, 46.8, 36.2, 29.4; ESI-MS (m/z): 370.1 [M - Br]+, 372.1 [M + 2 - Br]+, 373.1 [M + 3 - Br]+.
4d: Yield 833 mg (64%); mp 148-152 °C; IR (cm−1) 1609, 3378; 1H NMR (400 MHz, DMSO-d6) δ 13.52 (s, 1H), 9.36 (s, 1H), 8.74 (s, 1H), 7.80 (t, J = 1.8 Hz, 1H), 7.70 (t, J = 1.8 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.37–7.34 (m, 2H), 7.18–7.14 (m, 2H), 6.53–6.28 (m, 2H), 4.45 (t, J = 6.8 Hz, 2H), 4.12 (t, J = 5.8 Hz, 2H), 3.93 (s, 3H), 2.40–2.34 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.2, 163.1, 162.9, 162.1, 160.2, 144.8, 137.3, 134.6, 124.0, 123.5, 123.4, 122.9, 116.7, 116.5, 113.6, 107.5, 101.8, 65.4, 46.8, 36.2, 29.4; ESI-MS (m/z): 354.17 [M - Br]+.
4e: Yield 910 mg (68%); mp 140-145 °C; IR (cm−1) 1614, 3406; 1H NMR (400 MHz, DMSO-d6) δ 13.85 (s, 1H), 9.38 (s, 1H), 8.70 (s, 1H), 7.78 (t, J =1.8, 1H), 7.68 (t, J = 1.7, 1H), 7.40 (d, J = 8.3, 1H) 7.30 (td, J = 8.9, 2.16 Hz, 2H), 6.95 (td, J = 8.9, 2.16 Hz, 2H), 6.45–6.43 (m, 2H), 4.47 (t, J = 6.9 Hz, 2H), 4.11 (t, J = 5.8 Hz, 2H), 3.94 (s, 3H), 3.82 (s, 3H), 2.40-2.34 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.3, 162.5, 161.0, 158.6, 141.0, 137.3, 134.3, 124.0, 122.9, 122.8, 115.9, 115.1, 114.9, 113.7, 107.3, 101.8, 65.3, 55.8, 55.7, 46.8, 36.2, 29.4; ESI-MS (m/z): 366.2 [M - Br]+.
4f: Yield 699 mg (50%); viscous liquid; IR (cm−1) 1613, 3389; 1H NMR (400 MHz, DMSO-d6) δ 13.70 (s, 1H), 9.43 (s, 1H), 8.73 (s, 1H), 7.79 (t, J 1.9 Hz, 1H), 7.69 (t, J = 1.9 Hz, 1H), 7.74 (d, –J = 1H) 7.44–7.40 (m, 3H), 7.31–7.24 (m, 3H), 6.65–6.58 (m, 1H), 6.48–6.40 (m, 2H), 4.47 (t, J = 6.9 Hz, 2H), 4.12 (t, J = 5.8 Hz, 2H), 3.95 (s, 3H), 2.42–2.35 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 163.9, 163.4, 163.1, 145.7, 137.3, 134.7, 134.1, 128.5, 128.2, 127.0, 126.8, 124.1, 122.9, 122.8, 114.8, 114.1, 107.7, 101.9, 65.5, 46.8, 36.3, 29.4; ESI-MS (m/z): 386.2 [M - Br]+.
5 In vitro antibacterial assay
In vitro antibacterial activities were studied using the disc diffusion method [32] at a concentration of 128 μg mL−1 for all the tested compounds with DMSO as a solvent. For this purpose, Mueller–Hinton agar medium (HiMedia, India) was prepared and sterilized by autoclaving at 121 °C at 15 psi for 15 min. Medium was poured into sterile petri dishes (90 mm diameter) under aseptic conditions using laminar air flow chamber. After solidification of medium, the suspension of test organism (106 cfu mL−1) was swabbed on to individual media plates using a sterile glass spreader. A sterile disc (9 mm diameter) impregnated with compound was placed over media surface and the plates were incubated at 37 °C for 18 to 24 h under dark condition. Solvent (DMSO) was used as a negative control. Broad spectrum antibiotic chloramphenicol at a concentration of 30 μg mL−1 was used as a positive control to confirm the susceptibility of test organisms. Three replicates were maintained for each compound and their mean values were calculated.
MIC values were evaluated for all the compounds (4a-f) using broth macrodilution method as per the standard guidelines [33]. Experiments were carried out for all the compounds at 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, 128.0 μg mL−1 concentrations. A set of tubes containing Mueller–Hinton broth medium with different concentrations of compounds were prepared. The tubes were inoculated with bacterial cultures (106 cfu mL−1) and incubated on a rotary shaker at 37 °C for 18 to 24 hours under dark conditions. MIC value was defined as lowest concentration of compound that prevented the visible growth of bacteria after the incubation period. All the experiments were performed in three replicates.
6 In vitro antifungal assay
In vitro antifungal activities were determined by standard agar well diffusion method at a concentration of 128 μg mL−1 for all the synthesized compounds [34]. Autoclaved Potato Dextrose Agar (PDA) medium (HiMedia, India) was poured into sterile petri dishes (90 mm diameter) under aseptic conditions in a laminar air flow chamber. The medium was allowed to solidify and then inoculated with 100 μl of 3 days old fungal broth culture. After inoculation, wells of 9 mm diameter were prepared in petri dishes using sterile metallic borer. A drop of the soft agar was dropped into the well to seal the bottom. After allowing agar solidification for 10 minutes, 100 μl of compound solutions were added in respective wells and the plates were incubated at 28 °C for 4 days under dark conditions. DMSO and carbendazim (Methyl 1H-benzimidazol-2-ylcarbamate, a standard antifungal antibiotic) were used as negative and positive controls, respectively. Mean diameter of inhibition zone was measured to determine the magnitude of antifungal activity. The experiment was performed in three replicates.
MIC assay was performed to determine the lowest concentration of compound that prevents any discernible growth which can be detected visually. Assay was carried out for all the compounds at 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, 128.0 μg mL−1 concentrations. A set of tubes containing 10 mL of sterilized Czapek's dox broth medium (HiMedia, India) were inoculated with 100 μl of 3 days old fungal culture. Appropriate amount of compounds were added to achieve the desired concentrations. The tubes were incubated at 28 °C for 4 days and carefully observed for the presence of turbidity.
Acknowledgements
This work was financially supported by the Department of Science and Technology, New Delhi, India. We thank Regional Sophisticated Instrumentation Centre (RSIC), Chandigarh and Advanced Instrumentation Research Facility (AIRF), JNU, New Delhi for spectral analysis.


