1 Introduction
Multicomponent reactions (MCRs) have played vital roles in organic synthesis [1] and is considered as one of the premier methods for the quick building of novel and complex molecules including bioactive heterocyclic scaffolds with least number of steps [2–4].
Organophosphorus compounds are important synthetic targets in organic synthesis due to their wide range of biological significance. Among these, phosphorus containing compounds having substituents at α- and β-positions have gained much attention owing to the broad range of biological properties as enzyme inhibitors [5], metabolic probes [6], and peptide mimetics [7]. Additionally, 2-amino-4H-chromen-4-ylphosphonates are being used as anti-cancer [8], anti-inflammatory [9], anti-malarial [10] agents and are important constituents of various natural products [11]. Due to their wide biological applications, various routes have been developed for the synthesis of β-phosphonomalonates and 2-amino-4H-chromen-4-ylphosphonates. However, the domino Knoevenagel-phospha-Michael (DKPM) strategy, which involves the reaction between aromatic aldehyde, active methylene compound and phosphite ester has recently gained considerable attention. Variety of catalysts as clay-supported heteropolyacid [12], γ-Fe2O3-pyridine based catalyst [13], 3-aminopropylated silica gel [14], sodium stearate [15], HClO4–SiO2 [16], Fe-doped single walled carbon nanotubes [17], lanthanum(III) triflate supported on nanomagnetic γ-Fe2O3 [18], polystyrene-supported DABCO [19] pyridine-grafted graphene oxide [20], quaternary ammonium salt [H-dabco][AcO] [21], di-n-butylamine [22], nanosized zinc oxide [23], Phosphomolybdic acid [24], have been employed for the synthesis of β-phosphonomalonates. Further, catalysts like nano-MgO [25], sulfochitosan encapsulated nano-Fe3O4, [26] ionic liquid [Bmim]OH [27], PEG [28], potassium phosphate [29], β-cyclodextrin [30], InCl3 [31], and electrochemical approach [32] have been used for the synthesis of 2-amino-4H-chromen-4-ylphosphonates. Importantly, to the best of our knowledge, there are only few reports on the use of common catalysts [33–38] for the construction of both β-phosphonomalonates and 2-amino-4H-chromen-4-ylphosphonates scaffolds. Recently, DMAP has evolved as an efficient catalyst [39] due to its water tolerant property, accessibility at a modest price and well documented efficacy in many organic conversions such as Baylis–Hilman reaction [40], indole synthesis [41], and lactamization [42].
Microwave technology has witnessed an extensive popularity in the past few years due to its great efficiency in organic transformation with the introduction of greater molecular diversity in a short reaction time [43]. Reactions in aqueous condition offer several benefits as water is economical, non-toxic and shows immense selectivity [44]. Further, the combination of water as solvent and microwave heating, widely acknowledged as aqueous microwave- assisted chemistry has evolved into a rapid unconventional synthetic route strictly as per the principles of green chemistry [45].
2 Results and discussion
In view of our curiosity in developing novel synthetic routes using aqueous condition [46], we disclose herein a new route to synthesize β-phosphonomalonates and 2-amino-4H-chromen-4-ylphosphonates via the domino Knoevenagel-phospha-Michael reaction of benzaldehyde, malononitrile and triethylphosphite. We started our investigation by performing the model reaction of 1 mmol of each of benzaldehyde, malononitrile and triethylphosphite with 10 mol % DMAP in water at room temperature (Scheme 1).
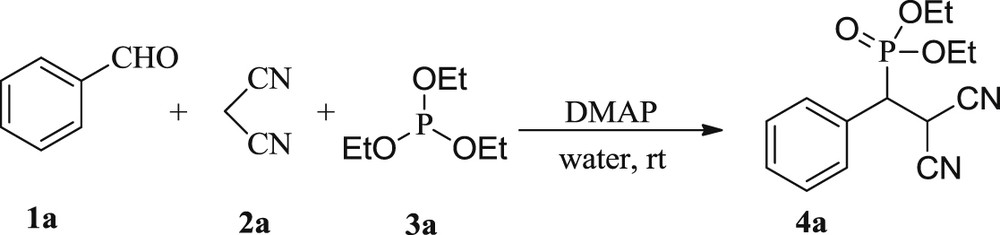
Synthesis of diethyl-(2,2-dicyano-1-phenylethyl) phosphonate.
The reaction provided the desired product, diethyl (2,2-dicyano-1-phenylethyl)phosphonate, 4a, which was isolated in 28% yield (20 h) as revealed by comparison of its physical and spectroscopic data [17]. Then we heated the reaction mixture under reflux to evaluate the effect of temperature and surprisingly the yield was increased to 45% (15 h). Next, we optimized catalyst charge by varying load of DMAP. Importantly, 20 and 30 mol % led to the improved conversion to afford the desired product in 88% and 89% (Table 1, entries 3 and 4), therefore, 20 mol % under reflux was selected for solvent screening. It is clear from Table 1 (entries 6–12) that the reaction successfully occurred both in solvents and under neat conditions, however with less yields. The water emerged as the best solvent as the reactions in aqueous conditions were carried out efficiently, thus avoiding the use of volatile and toxic organic solvents.
Optimization of catalysts and conditionsa.
| Entry | Catalyst (mol %) | Solvent | Temperature | Time (h) | Yield (%)b |
| 1 | DMAP (10) | H2O | r.t | 20 | 28 |
| 2 | DMAP (10) | H2O | reflux | 15 | 45 |
| 3 | DMAP (20) | H2O | reflux | 10 | 88 |
| 4 | DMAP (30) | H2O | reflux | 7 | 89 |
| 5 | no catalyst | H2O | reflux | 24 | trace |
| 6 | DMAP (20) | EtOH | reflux | 10 | 80 |
| 7 | DMAP (20) | MeOH | reflux | 10 | 78 |
| 8 | DMAP (20) | CH2Cl2 | reflux | 10 | 76 |
| 9 | DMAP (20) | CHCl3 | reflux | 10 | 78 |
| 10 | DMAP (20) | DMSO | reflux | 10 | 70 |
| 11 | DMAP (20) | THF | reflux | 10 | 70 |
| 12 | DMAP (20) | neat | 100 °C | 10 | 79 |
| 13 | DBU (20) | H2O | reflux | 10 | 82 |
| 14 | CSA (20) | H2O | reflux | 10 | 72 |
| 15 | Zn(Proline)2 (20) | H2O | reflux | 10 | 74 |
| 16 | l-proline 20 | H2O | reflux | 10 | 70 |
a Reactions conditions: 1 mmol of each of 1a, 2a and 3a in 1 mL of solvent.
b Yields are for isolated products.
Finally, related organocatalysts were screened for comparative purposes for 4a and results are depicted in Table 1 (entries 13–16). DMAP appeared to be better as compared to other examined catalysts. It is worth mentioning that without the catalyst, only trace amount of the product was detected even after 24 h (Table 1, entry 5).
With all the optimal reaction conditions in hand, additional substrates were screened, the reaction worked well with aldehydes bearing electron withdrawing and electron donating groups. Highest yield, 90% was obtained with 4-methoxybenzaldehyde, 4b (Table 2, entry 2) however, 4-nitrobenzaldehyde, 4c furnished relatively less yield, 75% (entry 3). Importantly, trimethylphosphite also coupled efficiently under optimized conditions providing the desired product, 4g in good yield (80%, entry 7). With ethyl cyanoacetate as active methylene partner, a comparatively low yield of the product, 4h was obtained (75%, entry 8). Pertinent to mention that a trace amount of knoevenagel product and α-hydroxy phosphonates generated from probable hydrophosphonation of aldehydes was also detected in some cases, however we have purified and isolated only a major product under the present protocol.
Substrate scope for β-phosphonomalonatesa.
| Entry | Product 4(a–h) | Time | Yield (%)b | mp (°C) | |||
| Reflux | mw | Reflux | mw | obs. | lit. | ||
| 1. | 12 h | 12 min | 88 | 92 | 55–56 | 53–54 [17] | |
| 2. | 8 h | 8 min | 90 | 95 | 59–60 | 60–62 [17] | |
| 3. | 16 h | 15min | 75 | 78 | 106 | 104–105 [17] | |
| 4. | 15 h | 14 min | 77 | 80 | 76 | 75–77 [17] | |
| 5. | 12 h | 12 min | 89 | 91 | 94 | 93–95 [17] | |
| 6. | 12 h | 12 min | 88 | 90 | 94 | 93–95 [17] | |
| 7. | 13 h | 14 min | 80 | 84 | 72 | 73 [47] | |
| 8. | 15 h | 13 min | 75 | 79 | Oil [21] | — |
a All reactions were carried out with 1 mmol of each of 1, 2 and 3 in 1 mL of water.
b Yields are for isolated products.
Fascinating with the well recognized applications of aqueous microwave-assisted technology [45], we irradiated the equimolar mixture of benzaldehyde, malononitrile and triethylphosphite at 100 °C in a microwave reactor (Biotage, Model: Initiator EXP EU 355301, 012180). We were pleased to note that the desired product, 4a was formed much faster (12 min) with improved yield from 88 to 92% (Table 4, entry 1). Based on this observation and for comparison purposes, we screened additional substrates and the results are summarised in Table 2. The improvement in terms of yields and time economy was observed in almost all the cases compared to the conventional route, which undoubtedly recognized the synergism between water and microwaves under the present study.
Synthesis of 2-amino-4H-chromen-4-yl-phosphonates under optimized conditionsa.
| Entry | Product 6(a–h) | Time | Yield (%)b | mp (°C) | |
| obs. | lit. | ||||
| 1. | 10 min | 95 | 139–141 | 140–142 [37a] | |
| 2. | 10 min | 89 | 151 | 156–158 [37a] | |
| 3. | 10 min | 90 | 150–151 | 148–150 [37a] | |
| 4. | 10 min | 87 | 167–169 | 166–168 [37a] | |
| 5. | 10 min | 90 | 177–178 | 177–178 [37a] | |
| 6. | 10 min | 88 | 159–161 | 160–161 [37a] | |
| 7. | 15 min | 84 | Oil [36b] | ||
| 8. | 15 min | 85 | Oil [36b] |
a All reactions were carried out with 1 mmol of each of 1, 2 and 3 in 1 mL of water.
b Yields are for isolated products.
Although it is tricky to quantitatively compare the efficiency of different catalysts, for 4b, our catalyst gave either a better or comparable yield to that reported in the literature (Table 3, entries1-8) with an additional advantage of time economy by combining microwave technology and aqueous conditions strictly in agreement with principles of green chemistry. Notably the catalysts (Table 3, entry 3, 5, 6, 8 and 9) were effective at lower loadings.
Comparative data of DMAP with previously reported catalysts for the synthesis of 4b.
| Entry | Catalyst (mol %) | Method | Time | Yield (%) |
| 1 | DMAP (20) | Microwavea | 8 min | 95b |
| 2 | DMAP (20) | Conventionala | 8 h | 90b |
| 3 | Diethylamine (10) | Conventional | 15 min | 95 [35] |
| 4 | Ethylenediamine diacetate(20) | Conventional | 3 h | 72 [34] |
| 5 | Silica-bonded 2-HEAA (1) | Conventional | 15 min | 81 [33] |
| 6 | HClO4–SiO2 (3) | Conventional | 3 h | 83 [16] |
| 7 | 3-Aminopropylated silica (30) | Conventional | 2 h | 85 [14] |
| 8 | Py-GO (3) | Conventional | 1 h | 73 [20] |
| 9 | PS-DABCO (3) | Conventional | 2.5 h | 88 [19] |
a Reactions were carried out with 1 mmol of each of anisaldehyde, malononitrile and triethyl phosphite in 1 mL of water at 100 °C.
b Yields are for isolated products.
To further maximize the synthetic potential of this protocol, equimolar concentrations of differently substituted salicylaldehyde, malononitrile/ethylcyanoacetate and triethyl/trimethyl-phosphite were exposed to the microwave reactor under optimized reaction conditions. To our delight, entirely different scaffolds, 2-amino-4H-chromen-4-ylphosphonates were rapidly constructed providing the products 6a–h in 84–95% yields as summarized in Table 4, with maximum yield obtained for 6a, 95% (Table 4, entry 1). However, the yield decreased (84%, entry 7) with the use of ethyl cyanoacetate as the active methylene partner in place of malononitrile, akin to that of our previous observation for the synthesis of β-phosphonates.
The proposed mechanism for the synthesis of 6a is shown in Scheme 2. Firstly, salicylaldehyde, 5a and malononitrile, 2a undergoes Knoevenagel condensation in the presence of DMAP to form I followed by intramolecular cyclization to form imino coumarin, II through the intramolecular Pinner reaction.
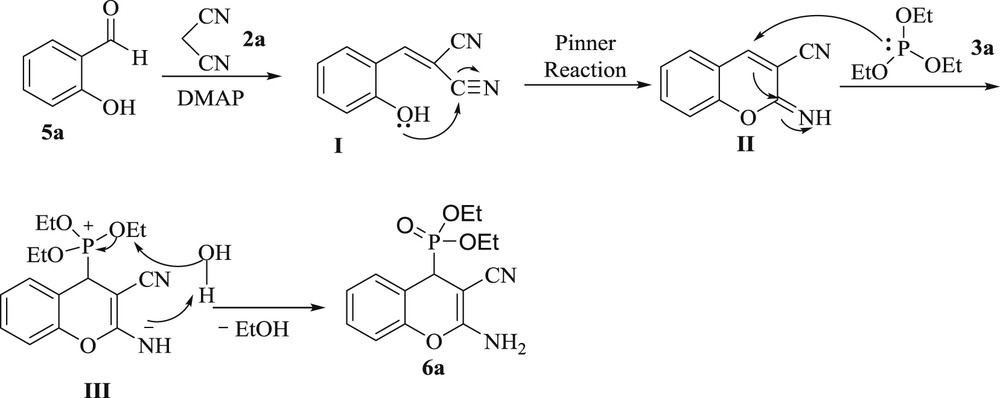
Proposed mechanism for the synthesis of 6a.
II upon further nucleophilic attack of triethylphosphite, 3a gives the intermediate, III, which finally react to provide, 6a.
3 Conclusion
In summary, we have established a DMAP catalyzed novel and expeditious route for the synthesis of β-phosphonomalonates and 2-amino-4H-chromen-4-ylphosphonates via a tandem Knoevenagel-phospha-Michael reaction by utilizing aqueous microwave chemistry. The presented protocol is simple, ecofriendly, high yielding and has undoubtedly recognized the synergism between water and microwaves.
Acknowledgements
We are highly thankful to IIIM, Jammu, for providing the spectra. P. K. thanks UGC, Govt. of India for a BSR fellowship.


