1 Introduction
Palladium commonly has been applied as one of the powerful metals for CC coupling reactions [1–3]. Cross-coupling reactions such as Heck and Suzuki reactions were used as significant procedures in modern synthetic organic chemistry for the preparation of natural products, pharmaceuticals, agrochemicals, herbicides, biologically active compounds, UV screens, polymers, hydrocarbons, liquid crystal materials and advanced materials [4–7]. Homogeneous Pd-catalysts have been widely reported in carbon–carbon coupling reactions because of their high catalytic activity [8], while, in the catalytic reactions, separation and reusability of the catalyst is an important factor because of stringent ecological and economical demands for sustainability [9–11]. Therefore, nanoparticles (NPs) have recently emerged as efficient alternatives for the immobilization of homogeneous catalysts [12,13]. Because, by decreasing the support size, the surface area is increased and consequently a semi-homogeneous media is obtained, which can be used as a bridge to improve the gap between homogeneous and heterogeneous catalysts [14]. However, in order to simplify catalyst recovery and reusability of the particles, magnetic nanoparticles (MNPs) have recently emerged in catalyst science, and they can be rapidly isolated from the reaction mixture using an external magnet [15]. More importantly, magnetic separation is more effective and easier than filtration or centrifugation [16]. Among the various magnetic nanoparticles, Fe3O4 MNPs have many advantages such as easy preparation, having a high surface-area, low toxicity, and easy availability [17,18]. Therefore, Fe3O4 MNPs are considered as ideal support for the heterogenization of homogeneous catalysts [10–13].
2 Result and discussion
2.1 Catalyst preparation
In continuation of our studies about the application of new catalysts in organic functional group transformations [16,19], herein we report a simple and efficient method for the carbon–carbon cross-coupling reaction in the presence of catalytic amounts of Pd–SMU-MNPs. The Pd–SMU-MNPs were prepared by the concise route that has been outlined in Scheme 1. Initially, naked Fe3O4 MNPs were prepared by coprecipitation of iron(II) and iron(III) ions in basic solution at 80 °C [16], followed by modification using (3-chloropropyl)trimethoxysilane (CPTES). Subsequently, S-methylisothiourea immobilized on Fe3O4 magnetic nanoparticles (SMU-MNPs) via the reaction of ClPrSiFe3O4 with a S-methylisothiourea hemisulfate salt. Finally, Pd–SMU-MNPs were prepared via coordination of palladium(II) with SMU-MNPs followed by reduction of Pd(II) to Pd(0) by NaBH4. The catalyst has been characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), vibrating sample magnetometry (VSM), inductively coupled plasma atomic emission spectroscopy (ICP-OES), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR).
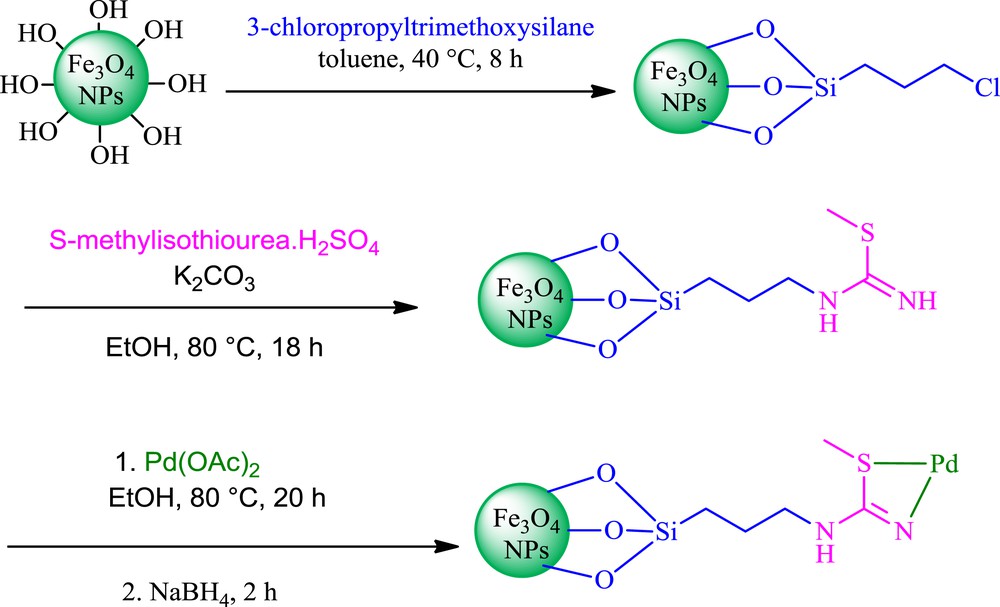
Preparation process of Pd–SMU-MNPs.
2.2 Catalyst characterization
The morphology and particle size of Pd–SMU-MNPs were evaluated by SEM and TEM analysis. The SEM image shows that these nanoparticles are spherical in shape with an average diameter of 24 ± 3 nm (Fig. 1). Also, TEM images reveal that the diameter of the Pd–SMU-MNPs is 15 ± 3 nm (Fig. 2).

SEM image of Pd–SMU-MNPs.
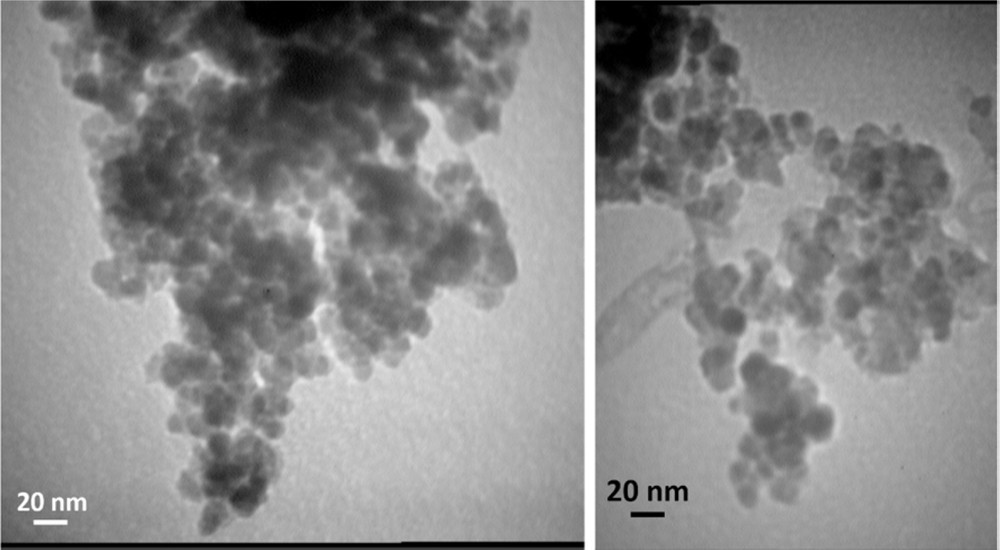
TEM images of Pd–SMU-MNPs.
In order to determine the exact amount of palladium in Pd–SMU-MNPs, the ICP-OES technique was performed. The amount of loaded palladium on the Pd–SMU-MNPs was found to be 1.54 × 10−3 mol g−1 based on inductively coupled plasma atomic emission spectroscopy (ICP-OES).
The XRD patterns of the Fe3O4 nanoparticles and catalyst showed several peaks at 2θ values of 30.5°, 35.8°, 43.6°, 54.3°, 57.5° and 63.2° related to crystal planes in the Fe3O4 cubic lattice (Fig. 3). Also the XRD pattern of Pd–SMU-MNPs shows a series of peaks (40.0°, 46.4° and 67.4°), and these results revealed that the surface modification of the Fe3O4 nanoparticles didn't destroy their cubic lattice structures [20].
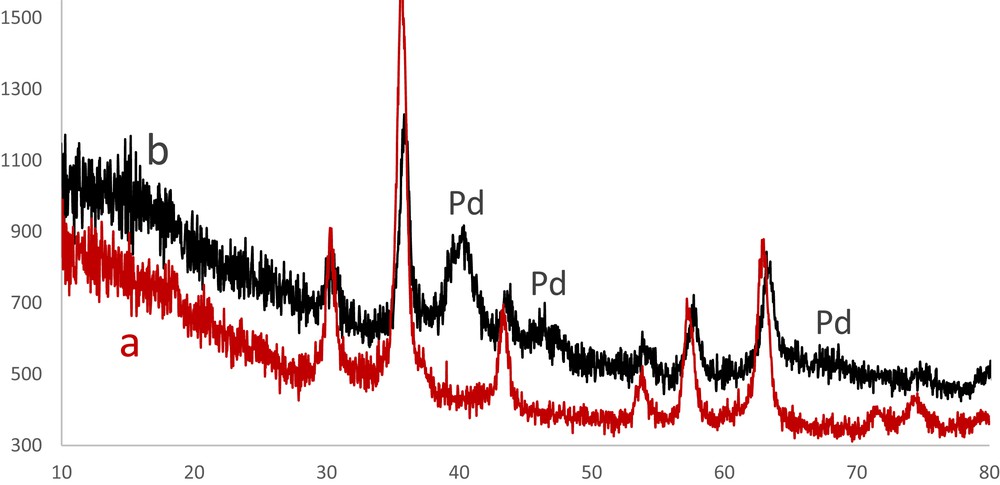
XRD patterns of Fe3O4 nanoparticles (a) and Pd–SMU-MNPs (b).
Successful functionalization of the Fe3O4 NPs can be inferred from the FT-IR technique. Fig. 4 shows FT-IR spectra of Fe3O4, Cl-MNPs, SMU-MNPs and Pd–SMU-MNPs. The FT-IR spectrum for bare Fe3O4 shows a stretching vibration at 3390–3440 cm−1 from both symmetrical and asymmetrical modes of OH bonds, which are attached to the surface of MNPs. The presence of Fe3O4 was shown in FT-IR by two strong absorption bands around 582 and 443 cm−1, which corresponds to the FeO bond of Fe3O4 [13]. The stretching vibrations at 990 cm−1 (corresponding to FeOSi bonds) and 1057 and 780 cm−1 (related to OSi bonds) indicate that the silica organic group was successfully coated on the surface of Fe3O4 nanocrystals [16,21]. Furthermore, the presence of the anchored organic groups including propyl silane is confirmed by CH stretching vibrations that appear at 2885 and 2973. Also, in the FT-IR spectrum of SMU-MNPs, the located bands around 1629 cm−1 correspond to the CN stretching vibrations, indicating that Cl of Cl-MNPs has been successfully replaced by S-methylisothiourea.
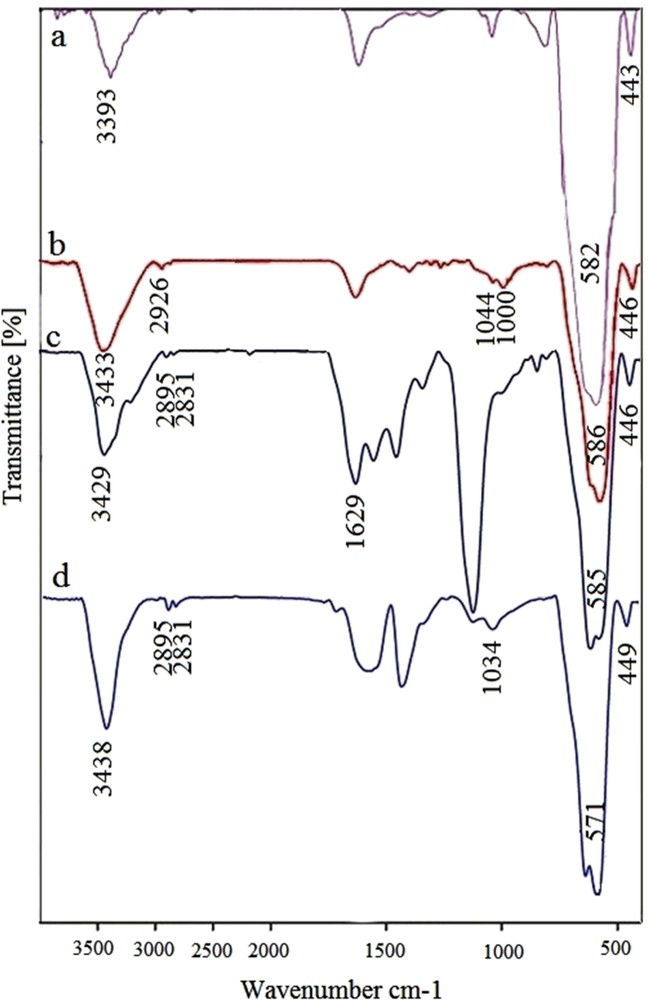
FT-IR spectra of Fe3O4 (a), Cl-MNPs (b), SMU-MNPs (c) and Pd–SMU-MNPs (d).
The superparamagnetic properties of particles were measured using the VSM technique. The magnetization curves of Fe3O4 MNPs and Pd–SMU-MNPs are shown in Fig. 5. The magnetic measurements show that Pd–SMU-MNPs have a saturated magnetization value of 42 emu g−1 and Fe3O4 MNPs have a saturated magnetization value of 74 emu g−1. Decreasing the magnetic properties of the Pd–SMU-MNPs (74 → 42 emu g−1), compared with the Fe3O4 MNPs is due to the coating of the Fe3O4 MNPs by organic layers and a palladium complex.
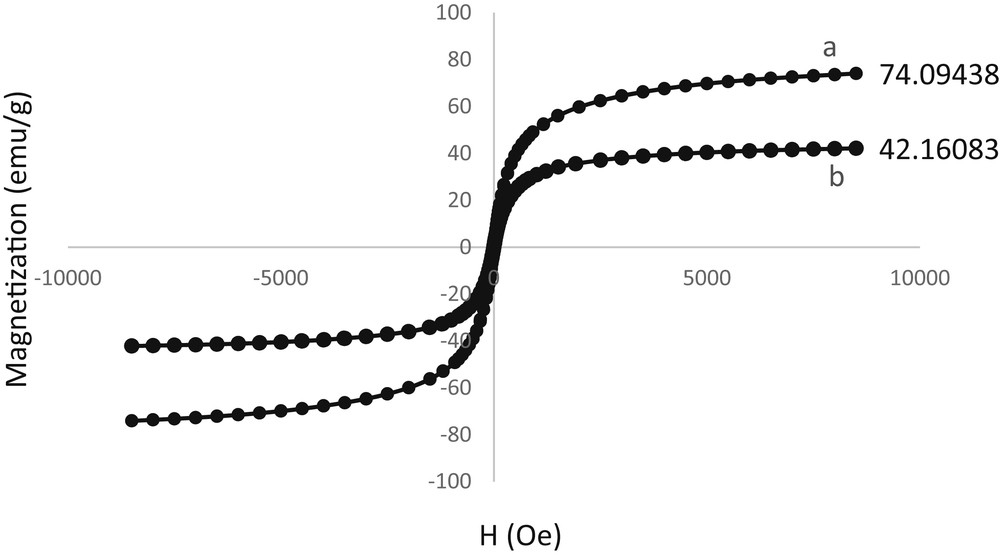
Magnetization curves of Fe3O4 (a) and Pd–SMU-MNPs (b) at room temperature.
2.3 Catalytic study
In order to study the catalytic activity of Pd–SMU-MNPs, Suzuki reaction has been performed using sodium tetraphenyl borate (NaBPh4) and phenylboronic acid (PhB(OH)2) in the presence of this nanostructural compound (Scheme 2).
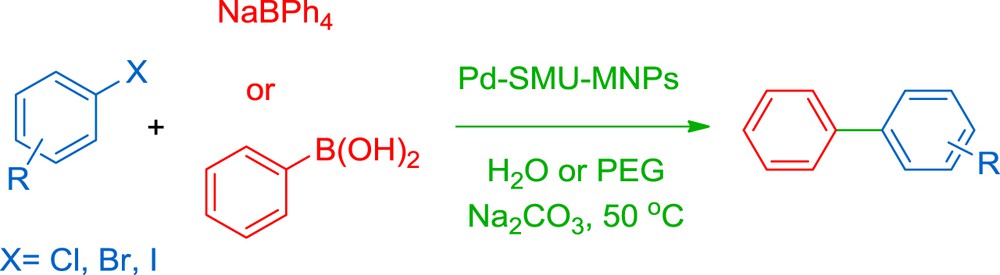
Pd–SMU-MNPs catalyzed the Suzuki reaction.
In order to optimize the reaction conditions, the effect of various parameters such as temperature, solvent (DMF, DMSO, toluene, water or PEG), bases (Et3N, KOH, NaOEt or K2CO3) and amount of catalyst were examined for the cross-coupling of iodobenzene with PhB(OH)2 (Table 1). We found that the reaction did not proceed in the absence of Pd–SMU-MNPs (Table 1, entry 9). As shown in Table 1, the best results were observed in the presence of 0.006 gr, 0.92 mol % of Pd–SMU-MNPs (Table 1, entry 4). Also, different bases and various solvents have been examined and the best results were obtained in H2O as solvent using 3 mmol of K2CO3 at 50 °C.
Optimization of reaction conditions for the CC coupling reaction of iodobenzene (1 mmol) with phenylboronic acid (1 mmol) in the presence of Pd–SMU-MNPs.
| Entry | Solvent (2 mL) | Base (3 mmol) | Catalyst (g) | Temperature (°C) | Time (min) | Yield %a |
| 1 | DMSO | K2CO3 | 0.006 (0.92 mol %) | 50 | 25 | 91 |
| 2 | DMF | K2CO3 | 0.006 (0.92 mol %) | 50 | 25 | 95 |
| 3 | Toluene | K2CO3 | 0.006 (0.92 mol %) | 50 | 30 | 43 |
| 4 | H2O | K2CO3 | 0.006 (0.92 mol %) | 50 | 30 | 96 |
| 5 | PEG | K2CO3 | 0.006 (0.92 mol %) | 50 | 30 | 97 |
| 6 | H2O | (Et)3N | 0.006 (0.92 mol %) | 50 | 30 | 56 |
| 7 | H2O | KOH | 0.006 (0.92 mol %) | 50 | 30 | 61 |
| 8 | H2O | NaOEt | 0.006 (0.92 mol %) | 50 | 30 | 18 |
| 9 | H2O | K2CO3 | – | 50 | 600 | –b |
| 10 | H2O | K2CO3 | 0.002 (0.31 mol %) | 50 | 30 | 37 |
| 11 | H2O | K2CO3 | 0.003 (0.46 mol %) | 50 | 30 | 49 |
| 12 | H2O | K2CO3 | 0.005 (0.75 mol %) | 50 | 30 | 68 |
| 13 | H2O | K2CO3 | 0.006 (0.92 mol %) | 40 | 30 | 55 |
| 14 | H2O | K2CO3 | 0.006 (0.92 mol %) | r.t. | 100 | 34 |
a Isolated yields.
b No reaction.
After optimization conditions, we examined the catalytic activity of Pd–SMU-MNPs for various aryl halides and the results are summarized in Table 2. In this study, various aryl iodides (Table 2, entries 1–3), bromides (Table 2, entries 4–10) and chlorides (Table 2, entries 11–13) were reacted with phenylboronic acid efficiently. All products were obtained in good yields. Therefore, this methodology is an efficient protocol for the cross-coupling a wide range of aryl halide including Cl, Br and I.
CC Coupling reaction of aryl halides (1 mmol) using PhB(OH)2 (1 mmol) or NaBPh4 (0.5 mmol) in the presence of Pd–SMU-MNPs (0.006 g, 0.92 mol %).
| Entry | Aryl halide | Phenylating reagent | Time (min) | Yield (%)a | Melting point (ºC) [Ref.] |
| 1 | Iodobenzene | Phenylboronic acid | 30 | 96 | 62–65 [22] |
| 2 | 4-Iodotoluene | Phenylboronic acid | 60 | 94 | 42–44 [22] |
| 3 | 2-Iodotoluene | Phenylboronic acid | 300 | 90 | Oil [23] |
| 4 | 4-Bromonitrobenzene | Phenylboronic acid | 20 | 99 | 115–117 [24] |
| 5 | 4-Bromochlorobenzene | Phenylboronic acid | 90 | 95 | 70–72 [23] |
| 6 | 4-Bromobenzonitrile | Phenylboronic acid | 60 | 96 | 82–84 [23] |
| 7 | Bromobenzene | Phenylboronic acid | 40 | 91b | 64–66 [22] |
| 8 | 4-Bromotoluene | Phenylboronic acid | 40 | 92b | 42–44 [22] |
| 9 | 4-Bromophenol | Phenylboronic acid | 40 | 92b | 161–163 [23] |
| 10 | 4-Bromobenzaldehyde | Phenylboronic acid | 70 | 88 | 55–57 [25] |
| 11 | 4-Chloronitrobenzene | Phenylboronic acid | 100 | 90b,c | 115–116 [24] |
| 12 | Chlorobenzene | Phenylboronic acid | 125 | 89b,c | 63–66 [22] |
| 13 | 4-Chlorobenzonitrile | Phenylboronic acid | 140 | 92b,c | 82–84 [23] |
| 14 | Iodobenzene | Sodium tetraphenyl borate | 30 | 97 | 64–66 [22] |
| 15 | 4-Iodotoluene | Sodium tetraphenyl borate | 45 | 94 | 43–44 [22] |
| 16 | 2-Iodotoluene | Sodium tetraphenyl borate | 280 | 91 | Oil [23] |
| 17 | 4-Bromonitrobenzene | Sodium tetraphenyl borate | 40 | 98 | 116–117 [24] |
| 18 | 4-Bromochlorobenzene | Sodium tetraphenyl borate | 20 | 99 | 70–72 [23] |
| 19 | 4-Bromobenzonitrile | Sodium tetraphenyl borate | 60 | 90 | 82–85 [23] |
| 20 | 4-Bromophenol | Sodium tetraphenyl borate | 60 | 90 | 161–163 [23] |
| 21 | 4-Bromobenzaldehyde | Sodium tetraphenyl borate | 100 | 89 | 56–58 [25] |
| 22 | 4-Chloronitrobenzene | Sodium tetraphenyl borate | 90 | 93b,c | 115–117 [24] |
| 23 | Chlorobenzene | Sodium tetraphenyl borate | 140 | 90b,c | 62–65 [22] |
| 24 | 4-Chlorobenzonitrile | Sodium tetraphenyl borate | 170 | 93b,c | 82–85 [23] |
a Isolated yield.
b PEG used as the solvent.
c Pd–SMU-MNPs was 8 mg, 1.2 mol %.
Also, the same reaction conditions have been applied for the cross-coupling of aryl halides with NaBPh4 in the presence of Pd–SMU-MNPs (Table 2, entries 14–24). Aryl halides including electron-neutral, electron-rich and electron-poor substituents reacted with NaBPh4 to produce the corresponding biphenyl in good to excellent yields (88–99% yield of products).
Also, we examined the catalytic activity of Pd–SMU-MNPs in the Heck reaction (Scheme 3). In order to find out the best reaction conditions, the coupling reaction of iodobenzene with butyl acrylate was selected as the model reaction (Table 3). The reaction conditions, such as the solvent (Table 3, entries 1–5), base (Table 3, entries 6–9), and amount of catalyst (Table 3, entries 9–13), were optimized in this model reaction (Table 3). Also, the effect of temperature (Table 3, entries 12–15) was examined. As shown in Table 3, the best conditions were obtained in the presence of Pd–SMU-MNPs (8 mg, 1.2 mol %) in DMF using K2CO3 at 120 °C (Table 3, entry 2).
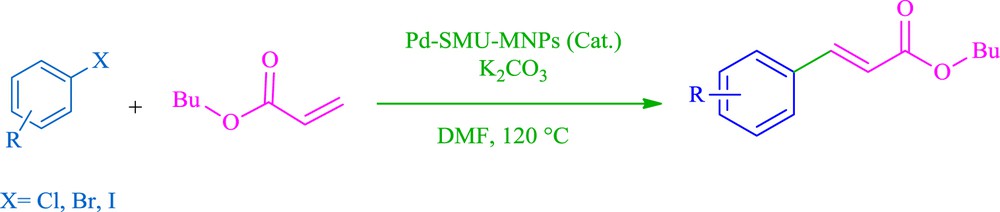
Pd–SMU-MNPs catalyzed the Heck reaction.
Optimization of reaction conditions for the CC coupling reaction of iodobenzene (1 mmol) with butyl acrylate (1.2 mmol) in the presence of Pd–SMU-MNPs.
| Entry | Solvent (2 mL) | Base (3 mmol) | Catalyst (g) | Temperature (°C) | Time (min) | Yield %a |
| 1 | DMSO | K2CO3 | 0.008 (1.2 mol %) | 120 | 90 | 30 |
| 2 | DMF | K2CO3 | 0.008 (1.2 mol %) | 120 | 90 | 95 |
| 3 | Toluene | K2CO3 | 0.008 (1.2 mol %) | 120 | 90 | 15 |
| 4 | DMSO:H2O | K2CO3 | 0.008 (1.2 mol %) | 120 | 90 | 45 |
| 5 | PEG | K2CO3 | 0.008 (1.2 mol %) | 120 | 90 | 60 |
| 6 | DMF | (Et)3N | 0.008 (1.2 mol %) | 120 | 90 | 65 |
| 7 | DMF | KOH | 0.008 (1.2 mol %) | 120 | 90 | 30 |
| 8 | DMF | NaOEt | 0.008 (1.2 mol %) | 120 | 90 | Trace |
| 9 | DMF | K2CO3 | – | 120 | 1200 | –b |
| 10 | DMF | K2CO3 | 0.004 (0.6 mol %) | 120 | 90 | 35 |
| 11 | DMF | K2CO3 | 0.005 (0.75 mol %) | 120 | 90 | 60 |
| 12 | DMF | K2CO3 | 0.007 (1.05 mol %) | 120 | 90 | 70 |
| 13 | DMF | K2CO3 | 0.008 (1.2 mol %) | 60 | 90 | 25 |
| 14 | DMF | K2CO3 | 0.008 (1.2 mol %) | 80 | 90 | 55 |
| 15 | DMF | K2CO3 | 0.008 (1.2 mol %) | 100 | 90 | 70 |
a Isolated yield.
b No reaction.
This optimized reaction conditions was then applied for the cross-coupling of different aryl halides including iodides (Table 4, entries 1–5), bromides (Table 4, entries 6–9) and chlorides (Table 4, entries 10–12) in the presence of Pd–SMU-MNPs and all products were obtained in good yield (91–97%). The reaction of various aryl halides (including electron-donating and electron-withdrawing groups) with butyl acrylate was investigated to confirm the generality of the present methodology.
Cross-coupling of aryl halides (1 mmol) with butyl acrylate (1.2 mmol) (Heck reaction) in the presence of catalytic amounts of Pd–SMU-MNPs (8 mg, 1.2 mol %).
| Entry | Aryl halide | Time (min) | Yield (%)a | Melting point (°C) [Ref.] |
| 1 | Iodobenzene | 90 | 96 | Oil [23] |
| 2 | 4-Iodotoluene | 120 | 95 | Oil [23] |
| 3 | 2-Iodotoluene | 350 | 91 | Oil [23] |
| 4 | 4-Iodoanisole | 90 | 93 | Oil [23] |
| 5 | 2-Iodoanisole | 450 | 92 | Oil [26] |
| 6 | 4-Bromonitrobenzene | 90 | 97 | 60–62 [23] |
| 7 | 4-Bromochlorobenzene | 720 | 91 | Oil [27] |
| 8 | 4-Bromobenzonitrile | 75 | 95 | 40–42 [27] |
| 9 | Bromobenzene | 80 | 95 | Oil [23] |
| 10 | Chlorobenzene | 300 | 93 | Oil [23] |
| 11 | 4-Chlorobenzonitrile | 420 | 90 | 39–42 [27] |
| 12 | 4-Chloronitrobenzene | 330 | 91 | 60–63 [23] |
a Isolated yields.
2.4 Recyclability of the catalyst
The reusability of catalysts is an important advantage from an industrial point of view. Therefore, the recovery and recyclability of Pd–SMU-MNPs were examined in the coupling reaction of iodobenzene with phenylboronic acid. After completion of the reaction, the catalyst was rapidly isolated from the reaction mixture by magnetic decantation and washed with diethyl ether to remove residual organic materials. Then, the reaction vessel was charged with fresh substrates and subjected to the next run. As shown in Fig. 6, the catalyst can be reused over eight times without any significant loss of its catalytic activity or palladium leaching. The average isolated yield for eight runs was 94.5%, which clearly proved the practical recyclability of this catalyst.
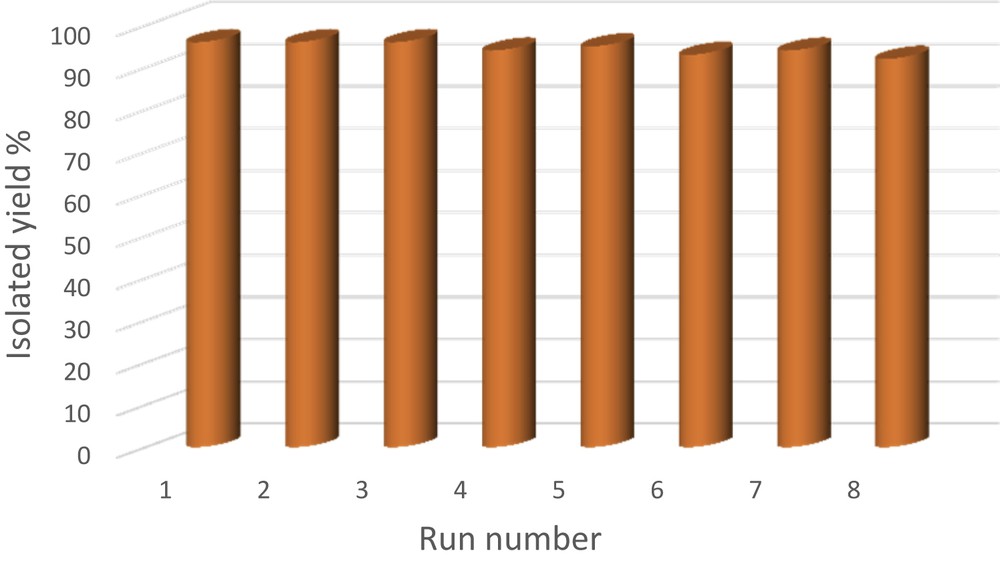
Recyclability of Pd–SMU-MNPs in the coupling of iodobenzene with phenylboronic acid.
In order to show that Pd–SMU-MNPs act heterogeneously in the reaction media, a hot filtration test was performed in the coupling reaction of iodobenzene with butyl acrylate. In this study, we obtained the yield of product in the half time of the reaction that was 63%. Then the reaction was repeated and in half time of the reaction, the catalyst separated and allowed the filtrate to react further. The yield of reaction in this stage was 64% confirming that the Pd–SMU-MNPs act heterogeneously in the reaction media.
Also, to determine the exact leaching of palladium in the catalyst, the amount of Pd in Pd–SMU-MNPs was determined by ICP-OES after six times recycling. The amount of Pd in the recovered catalyst was found to be 1.49 × 10−3 mol g−1 based on ICP-OES. Therefore the amount of Pd in the catalyst after six times recovery is comparable with the fresh catalyst (1.54 × 10−3 mol g−1 for fresh Pd–SMU-MNPs). Therefore, only 3% leaching of palladium from the catalyst was observed after six runs. The results from the hot filtration test and ICP-OES technique showed that leaching of palladium during the reaction is negligible.
Also, in order to determine leaching of palladium in the reaction mixture, the Suzuki reaction through the coupling of iodobenzene with phenylboronic acid was performed and the obtained biphenyl has been characterized by ICP-OES to detect amounts of palladium in products. Interestingly, in this analysis even trace amounts of palladium weren't observed in the biphenyl product.
2.5 Comparison of the catalyst with previously reported ones
In order to examine the efficiency of the described procedure, we compared the results of the coupling of iodobenzene with phenylboronic acid with those of the previously reported procedures in the literature (Table 5). This catalyst showed a shorter reaction time and higher reaction yield than the other catalysts. Also Pd–SMU-MNPs are superior in terms of price, bio-compatibility, stability and easy separation than the previously reported works. In addition, the recoverability and recyclability of Pd–SMU-MNPs is more rapid and easier than the other catalysts. As is evident Pd–SMU-MNPs (in this work) have many advantages such as a low reaction time, high number of TNO and TOF, high yield of products, reacting at low temperature and use of water as the solvent.
Comparison results of Pd–SMU-MNPs with other catalysts for the coupling of iodobenzene with phenylboronic acid.
| Entry | Catalyst (mol % of Pd) | Size of catalyst (nm) | Condition | Temperature (°C) | Time (min) | Yield (%)a | TON | TOF (h−1) | Ref. |
| 1 | NHC–Pd(II) complex (1.0 mol %) | – | THF, Cs2CO3 | 80 °C | 12 h | 88 | 88 | 7.33 | [28] |
| 2 | Pd NP (1.0 mol %) | 2.5–14 | H2O, KOH | 100 °C | 12 h | 95 | 95 | 7.92 | [29] |
| 3 | CA/Pd(0) (0.5–2.0 mol %) | 3.2 ± 0.4 | H2O, K2CO3 | 100 °C | 120 | 94 | 188 | 47 | [30] |
| 4 | Pd/Au NPs (4.0 mol %) | 80 | EtOH/H2O, K2CO3 | 80 °C | 24 h | 88 | 22 | 0.92 | [31] |
| 5 | Pd(II)–NHC complex (1 mol %) | – | DMF, Cs2CO3 | 100 °C | 24 h | 99 | 99 | 4.12 | [32] |
| 6 | N,N′-Bis(2-pyridinecarboxamide)-1,2-benzene palladium complex (1 mol %) | 10–20 | H2O, K2CO3 | 100 °C | 180 | 97 | 97 | 32.33 | [33] |
| 7 | Pd-MPTAT-1 (0.02 g) | 50 | NaOH, DMF: H2O (1:5) | 85 °C | 8 h | 95 | – | – | [34] |
| 8 | LDH-Pd(0) (0.3 g) | 6–10 | K2CO3, 1,4-dioxane: H2O (5:1) | 80 °C | 10 h | 96 | – | – | [35] |
| 9 | PANI-Pd (2.2 mol %) | 150–300 | K2CO3, 1,4-dioxane: H2O (1:1) | 95 °C | 240 | 91 | 41.36 | 10.34 | [36] |
| 10 | Pd–SMU-MNPs (0.006 mg, 0.92 mol %) | 15 ± 3 | H2O, K2CO3 | 50 °C | 30 | 96 | 104 | 208 | This work |
a Isolated yield.
3 Experimental
3.1 Preparation of the catalyst
The Fe3O4 MNPs were synthesized according to our recently reported procedure via a chemical coprecipitation technique using FeCl3·6H2O and FeCl2·6H2O in basic solution at 80 °C [23]. The obtained Fe3O4 nanoparticles (1.5 g) was dispersed in 50 mL toluene by sonication for 30 min, then 2.5 mL of (3-choloropropyl)triethoxysilane (CPTES) was added to the mixture. The reaction mixture was stirred under a N2 atmosphere at 40 °C for 8 h. Then, the prepared nanoparticles (Cl–MNPs) were washed with ethanol, separated by magnetic decantation and dried at room temperature. In the next step, the obtained Cl-MNPs (1 g) were dispersed in 50 mL ethanol for 20 min, then S-methylisothiourea hemisulfate salt (2.5 mmol) and potassium carbonate (2.5 mmol) were added to the reaction mixture and stirred for 24 h at 80 °C under a N2 atmosphere. Then, the resulting nanoparticles (SMU-MNPs) were washed with ethanol and separated using magnetic decantation and dried at room temperature. The obtained SMU-MNPs (1 g) were dispersed in 25 mL ethanol by sonication for 20 min; subsequently, palladium acetate (0.5 g, 1.95 × 10−3 mol) was added to the reaction mixture. The reaction mixture was stirred at 80 °C for 20 h. Then, NaBH4 (0.5 mmol) was added to the reaction mixture and it was continued for two more hours. The final product (Pd–SMU-MNPs) was separated by magnetic decantation, washed with ethanol and dried at room temperature.
3.2 General procedure for the Suzuki reaction
A mixture of aryl halide (1 mmol), phenylboronic acid (1 mmol) or sodium tetraphenyl borate (0.5 mmol), K2CO3 (3 mmol), and Pd–SMU-MNPs (0.006 g, 0.92 mol %) was added to a reaction vessel. The resulting mixture was stirred in H2O or PEG-400 at 50 °C and the progress of the reaction was monitored by TLC. After completion of the reaction, the catalyst was separated by an external magnet and washed with ethylacetate. The reaction mixture was extracted with H2O and ethylacetate and the organic layer was dried over anhydrous Na2SO4 (1.5 g). Then the solvent was evaporated and pure biphenyl derivatives were obtained in good to excellent yields.
3.3 General procedure for the Heck reaction
A mixture of aryl halide (1 mmol), n-butyl acrylate (1.2 mmol), K2CO3 (3 mmol), and Pd–SMU-MNPs (0.008 g, 1.2 mol %) was stirred in DMF at 120 °C (the progress of the reaction was monitored by TLC). After completion of the reaction, the mixture was cooled down to room temperature and the catalyst was separated by an external magnet, washed with diethyl ether and the reaction mixture was extracted with H2O and diethyl ether. The organic layer was dried over Na2SO4 (1.5 g); the solvent evaporated and pure products were obtained in 81–98% yields.
3.4 Selected spectral data
3.4.1 1,1′-Biphenyl
1H NMR (400 MHz, CDCl3): δH = 7.66–7.64 (m, 4H), 7.52–7.41 (m, 4H), 7.42–7.38 (tt, J = 7.6, 1.2 Hz, 2H) ppm.
3.4.2 [1,1′-Biphenyl]-4-carbonitrile
1H NMR (400 MHz, CDCl3): δH = 7.79–7.76 (m, 2H), 7.74–7.71 (m, 2H), 7.65–7.62 (m, 2H), 7.55–7.51 (m, 2H), 7.49–7.45 (m, 1H) ppm.
3.4.3 4-Chloro-1,1′-biphenyl
1H NMR (400 MHz, CDCl3): δH = 7.61–7.59 (m, 2H), 7.58–7.55 (m, 2H), 7.51–7.44 (m, 4H), 7.43–7.39 (tt, J = 6, 1.6 Hz, 1H) ppm.
3.4.4 Butyl cinnamate
1H NMR (400 MHz, CDCl3): δH = 7.75–7.71 (d, J = 16 Hz, 1H), 7.58–7.56 (m, 2H), 7.44–7.41 (m, 3H), 6.55–6.47 (d, J = 16 Hz, 1H), 4.27–4.24 (t, J = 6.4 Hz, 2H), 1.77–1.71 (m, 2H), 1.51–1.46 (m, 2H), 1.03–0.99 (t, J = 7.6 Hz, 3H) ppm.
3.4.5 Butyl 3-(p-tolyl)acrylate
1H NMR (400 MHz, CDCl3): δH = 7.72–7.68 (d, J = 16 Hz, 1H), 7.48–7.46 (d, J = 8 Hz, 2H), 7.24–7.22 (d, J = 8 Hz, 2H), 6.46–6.42 (d, J = 16 Hz, 1H), 4.26–4.23 (t, J = 13.2 Hz, 2H), 2.42 (s, 3H), 1.77–1.70 (quint, J = 6.4 Hz, 2H), 1.51–1.45 (sixt, J = 7.6 Hz, 2H), 1.03–0.99 (t, J = 7.6 Hz, 3H) ppm.
3.4.6 Butyl 3-(4-methoxyphenyl)acrylate
1H NMR (400 MHz, CDCl3): δH = 7.69–7.65 (d, J = 15.6 Hz, 1H), 7.52–7.50 (d, J = 6.8 Hz, 2H), 6.94–6.93 (d, J = 7.2 Hz, 2H), 6.37–6.33 (d, J = 16 Hz, 1H), 4.25–4.22 (t, J = 6.4 Hz, 2H), 3.86 (s, 3H), 1.74–1.68 (quint, J = 6.8 Hz, 2H), 1.52–1.43 (sixt, J = 8 Hz, 2H), 1.02–0.98 (t, J = 7.6 Hz, 3H) ppm.
4 Conclusion
In conclusion, an efficient heterogeneous nanocatalyst (Pd–SMU-MNPs) was synthesized by immobilization of palladium on modified Fe3O4 nanoparticles. This catalyst was characterized by FT-IR, SEM, TEM, XRD, VSM and ICP-OES techniques. The Pd–SMU-MNPs exhibit an excellent catalytic activity, high reusability and air or moisture stability for the Suzuki and Heck reactions. This methodology is effective for a wide range of aryl halide including Cl, Br and I. Also, the catalyst can be recovered and recycled over eight times and used without any significant loss of its activity or palladium leaching.
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.


