1 Introduction
The recovery of expensive catalysts after catalytic reactions and reusing them without loss of their chemical activity are important factors for sustainable process management [1,2]. The difficulty in separating homogeneous catalysts from the reaction medium restricts catalyst recycling in industries, particularly in drug and pharmaceutical industries, owing to the issue of metal contamination in the cases of metal-catalyzed synthesis [3]. Surface functionalization of magnetic nanoparticles is a well-designed method to bridge the gap between heterogeneous and homogeneous catalysis [4]. Since such catalysts work under quasi-homogeneous conditions, their performance typically exhibits high selectivity, activity, and stability. In many cases, catalysts can be easily separated by magnetic decantation and reused [5,6].
The ring opening of epoxides' reactions with different nucleophiles are important reactions in organic synthesis because the 1,2-disubstituted products are often useful and valuable intermediates. A literature survey indicated that a variety of catalytic methods under either basic or acidic conditions have been developed using the ring opening of epoxides with alcohols that leads to β-alkoxy alcohols. However, many catalytic systems have significant drawbacks, such as tedious work-up procedures or hazardous chemical waste products, which prompted organic researchers to develop a simple, environmentally benign method [7–11].
As a part of our ongoing investigations into sustainable synthetic methods [12–16], we recently introduced new magnetically recoverable nanocatalyst systems for organic reactions [17,18]. In this study, we report on a synthesis approach, structural characterization, and catalytic ability of a novel nanoparticle supported azo Schiff base.
Schiff bases are present as reactants in many synthetic organic processes, as important scaffolds in organometallic chemistry, as backbones of precious catalysts and as pharmaceutical presidiums against a series of different diseases and pathological states [19].
2 Experimental
2.1 General
All chemicals were purchased from Merck, Aldrich and Fluka and used without further purification. TLC was performed on aluminum-backed silica gel 60 F254 plates (0.2 mm thickness, Merck). Atomic absorption measurements were carried out on a Shimadzu AA-6300 spectrometer with a deuterium lamp background corrector. SEM-EDAX analyses were carried out using a Philips XL30 instrument. The TEM image was recorded using a Zeiss-EM10C-100 KV. Magnetism analysis was performed on a vibrating sample magnetometer (4 inch, Daghigh Meghnatis Kashan Company, Kashan, Iran) at room temperature. The FT-IR spectra were recorded using a BOMEM MB-Series 1998 FT-IR spectrometer.
2.2 Preparation of Mn2+-azo ligand@Am-SiO2@Fe3O4
First, 2 g of SiO2@Fe3O4 was dispersed in 10 mL of dry toluene and, sonicated for 15 min using an ultrasonic bath. Then, 2 mL of (3-aminopropyl) triethoxysilane was added. The mixture was stirred and refluxed for 72 h under a nitrogen atmosphere. The particles obtained were filtered off, washed with ethanol and diethyl ether and dried under reduced pressure to obtain Am-SiO2@Fe3O4.
Next, using the procedure described by Uruş et al. with a minor modification [20], 1 g of Am-SiO2@Fe3O4 was added to a solution of 0.121 g, 1 mmol of (Z)-5-({2,4-dichloro-6-hydroxyphenyl}diazenyl)-2-hydroxybenzaldehyde in 10 mL of ethanol. The mixture was stirred under reflux conditions for 12 h. The resultant MNPs were removed by using an external magnet and washed several times with ethanol and dried well to generate Azo ligand@Am-SiO2@Fe3O4.
Finally, to synthesis Mn2+-azo ligand@Am-SiO2@Fe3O4 nanoparticles, a mixture of 2.0 g Azo ligand@Am-SiO2@Fe3O4 in 10 mL of ethanol was sonicated for 15 min. Then, 0.173 g, 1 mmol of manganese acetate was added, and the resulting mixture was refluxed for 24 h. After removing the particles magnetically, the nanocomposites were washed with water and dried under reduced pressure.
2.3 Alcoholysis procedure
A mixture of epoxide (1 mmol), alcohol (5 mL) and Mn2+-azo ligand@Am-SiO2@Fe3O4 (0.05 g) was heated under reflux conditions for an appropriate time. After completion of the reaction monitored by GC or TLC analysis, the reaction mixture was cooled to room temperature and the magnetic nanocatalyst was separated by using an external magnet and washed with acetone for recycling experiments.
3 Results and discussion
The synthetic method employed to graft the azo Schiff ligand onto the silica-coated Fe3O4 and chelating ligands via nitrogen and oxygen atoms to the manganese center is outlined in Scheme 1. The first stage began with amination of the SiO2@Fe3O4 surface using 3-(azidopropyltrimethoxy)silane (APTES) in toluene under reflux conditions. Am-SiO2@Fe3O4 nanoparticles were subjected to a reaction with ethanol solution of the azo compound [(Z)-5-({2,4-dichloro-6-hydroxyphenyl}diazenyl)-2-hydroxybenzaldehyde]. The azo Schiff base was synthesized according to the reported procedure [21]. The ligand performed two roles: forming a complex with Mn(II) and enhancing the lipophilicity of the catalyst. Treatment of the latter nanoparticles with Mn(OAc) in ethanol under reflux conditions led to the target nanocomposites.
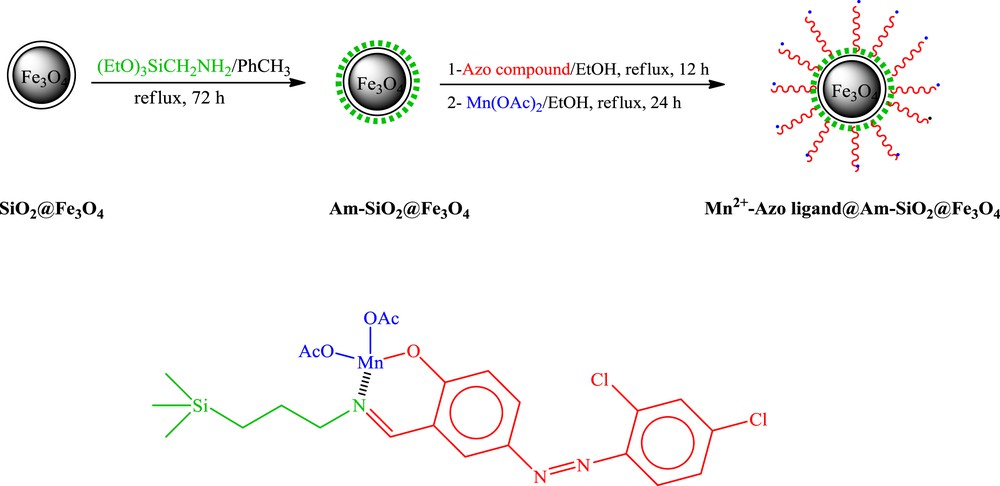
Preparation of Mn2+-azo ligand@Am-SiO2@Fe3O4 nanoparticles.
The structure analysis of the resultant Mn2+-azo ligand@Am-SiO2@Fe3O4 magnetic nanoparticles was then accomplished using FT-IR, SEM, EDX and VSM techniques (see Fig. 1).
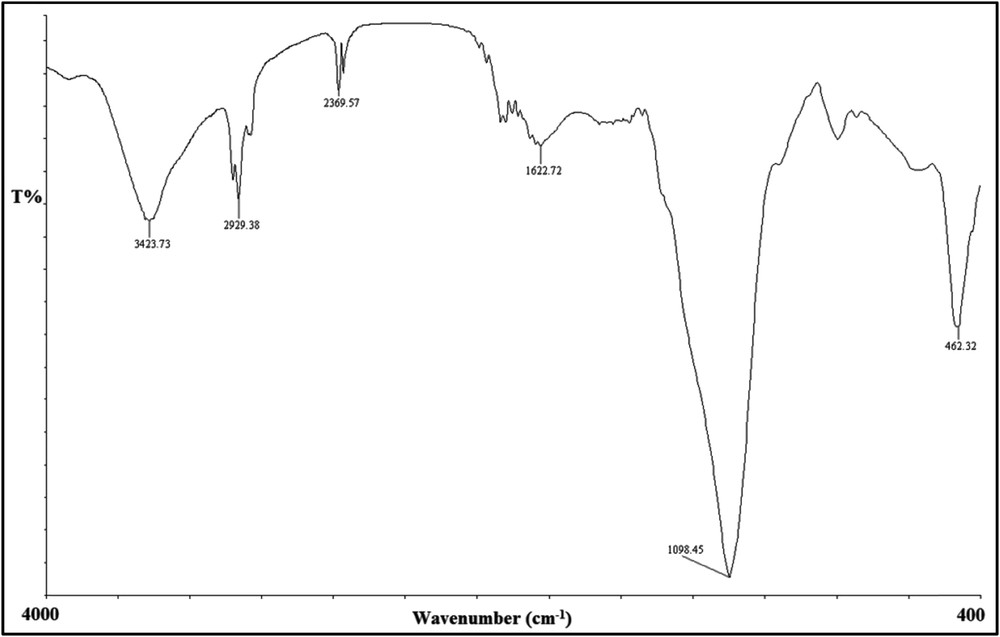
The FT-IR spectrum of Mn2+-azo ligand@APTES-SiO2@Fe3O4.
The FT-IR spectrum showed several peaks corresponding to the Fe3O4 core, SiO2 shell and azo Schiff ligand. The peak at ∼580 cm−1 was due to FeO vibration, and a broad peak at ∼3423 cm−1 was attributed to the SiOH group. Also, a strong peak at ∼1098 cm−1 belonged to antisymmetric stretching vibrations of the Si O Si bond. The peaks at ∼3100–2850 cm−1, ∼1622 cm−1 and ∼1480 cm−1 corresponded to vibration of the CH (aromatic rings and linker moiety), CC, and NN bonds, respectively.
The SEM and TEM images displayed a nearly spherical morphology and the nanosize of the Mn2+-azo ligand@Am-SiO2@Fe3O4 particles (Fig. 2).

SEM (left) and TEM (right) images of the nanoparticles.
The EDX imaging of the Mn (II)-azo Schiff complex grafted to Fe3O4 confirmed the presence of oxygen (33.2% wt), carbon (23.0% wt), silicon (19.7% wt), iron (12.6% wt), nitrogen (7.0% wt), chlorine (2.5% wt) and manganese (1.9% wt) (Fig. 3). The molar ratio of Mn/Cl is 0.5, which is compatible with the proposed structure.
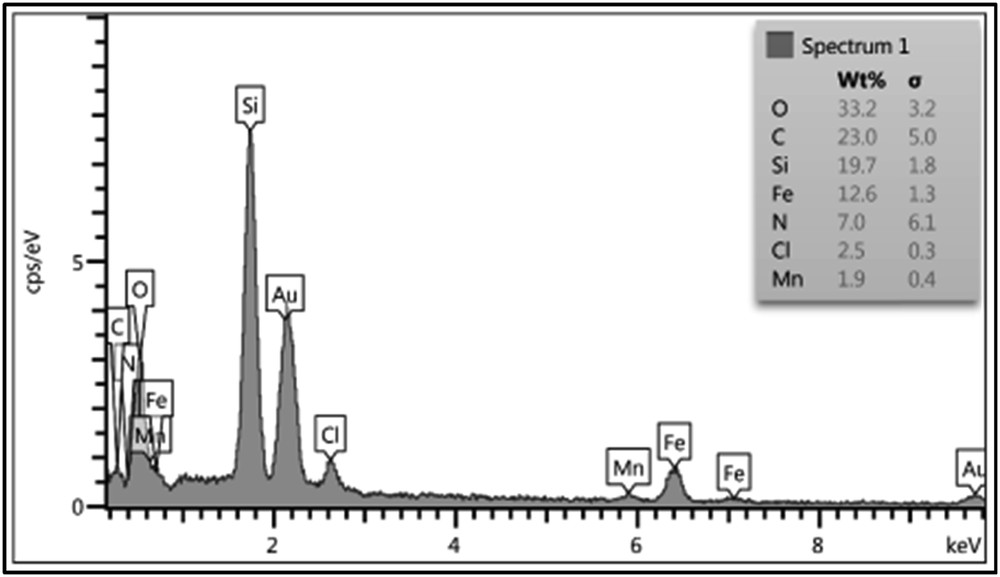
The energy-dispersive X-ray spectroscopy (EDX) of Mn2+-azo ligand@APTES-SiO2@Fe3O4.
The magnetic properties of Mn2+-azo ligand@Am-SiO2@Fe3O4 were also investigated at room temperature using a vibrating sample magnetometer (Fig. 4). Comparing the results of the neat Fe3O4 with Mn2+-azo ligand@Am-SiO2@Fe3O4, the saturation magnetization value decreases from 60 emu/g to about 15 emu/g. This decrease was due to the coating of the magnetite particles with non-magnetic materials: the SiO2 shell, linker moiety and azo Schiff base ligands. However, the catalyst could still be simply separated from the solution by applying an external magnetic force, though the saturation magnetization had been decreased (Fig. 5). The catalytic activity of the Mn loaded nanoparticles was investigated for the ring opening of epoxides with alcohols. First, treatment of 1 mmol of 2-(phenoxymethyl)oxirane with 5 mL of methanol, as a substrate and solvent, was chosen as a test reaction. The optimal reaction conditions were found to occur with 0.05 g of the magnetic nanocatalyst heated during the solvent refluxing. In addition, we did not detect conversion of the substrate in the absence of the catalyst or free Mn loaded nanoparticles (Azo ligand@Am-SiO2@Fe3O4). Accordingly, a range of epoxides was subjected to a reaction with methanol, ethanol and n-propanol in the presence of Mn2+-azo ligand@Am-SiO2@Fe3O4. For all cases, Table 1 reveals that a complete conversion of only one isomer was obtained which is the regiochemical outcome attack on the less substituted carbon of the aliphatic epoxides, with the exception of styrene epoxide. In this aryl epoxide, the major isomer resulted from an attack on the benzylic position (entries 1 and 3). To clarify the efficiency of the catalyst, the turnover number (TON) and turn over frequency (TOF) were calculated using the 0.34 mmol/g Mn obtained by EDAX, which are 58.8 and 6.5–39.2 h−1, respectively.
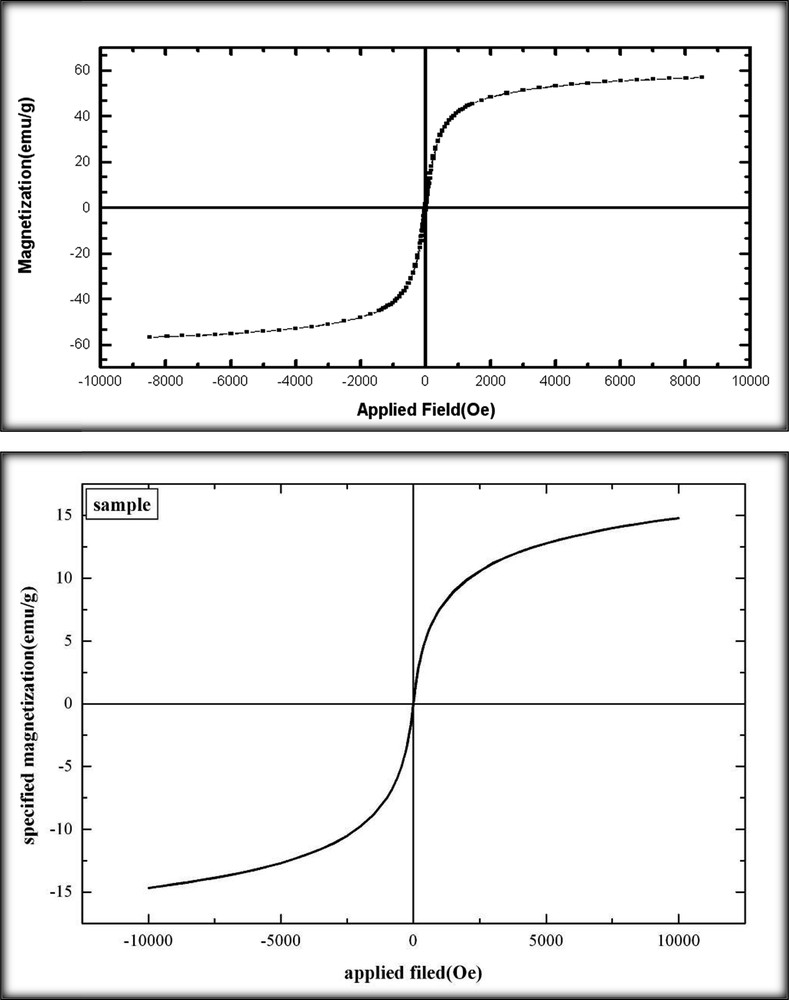
Room-temperature magnetization curves of Fe3O4 (upper) and Mn2+-azo ligand@APTES-SiO2@Fe3O4 (lower).

Removing the catalyst from the reaction mixture by magnetic separation.
Ring opening reaction with alcohols catalyzed by Mn2+-azo ligand@Am-SiO2@Fe3O4 nanoparticles.
| Entry | Epoxide | Alcohol | Product (s) | Time (h) | Conv.(%)a |
| 1 | CH3OH | 6 | 60/40 | ||
| 2 | EtOH | 9 | 100 | ||
| 3 | n-Propanol | 7 | 75/25 | ||
| 4 | CH3OH | 2.5 | 100 | ||
| 5 | EtOH | 3 | 100 | ||
| 6 | n-Propanol | 2 | 100 | ||
| 7 | CH3OH | 2 | 100 | ||
| 8 | EtOH | 3 | 100 | ||
| 9 | n-Propanol | 2 | 100 | ||
| 10 | CH3OH | 4 | 100 | ||
| 11 | EtOH | 2 | 100 | ||
| 12 | n-Propanol | 2.5 | 100 | ||
| 13 | CH3OH | 2.5 | 100 | ||
| 14 | EtOH | 3 | 100 | ||
| 15 | n-Propanol | 1.5 | 100 |
a Determined by GC.
The development of recoverable and recyclable catalysts represents a fascinating challenge for the industry [22]. Interestingly, the nanomagnetic catalyst could be reused at least five times without loss of activity in the synthesis reaction of 1-methoxy-3-phenoxypropan-2-ol (Fig. 6, conversion decreases from 100% to 92%).
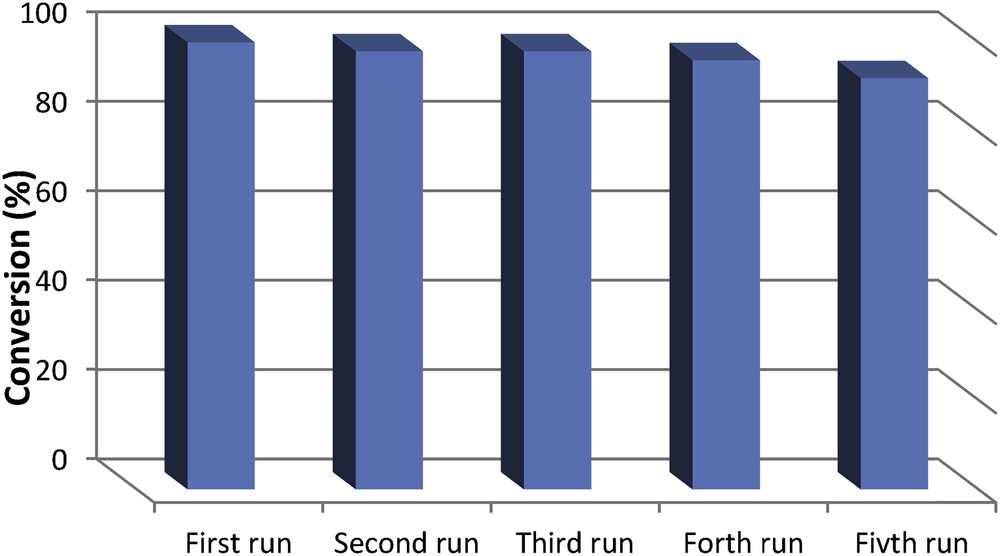
Recycling of the nanocatalyst.
When using a supported catalyst, a crucial issue is the possibility that some of the active sites could migrate from the solid support to the liquid phase, where these leached species could become responsible for a significant portion of the catalytic activity [23]. In order to investigate this issue, a mixture of the catalyst (0.5 g) and methanol was refluxed for 12 h. Then the nanoparticles were carefully separated by using an external magnet. A sample of the solution was then analyzed by an atomic absorption technique, and less than 1 ppm of Mn was detected.
4 Conclusion
In summary, a new magnetite-supported organocatalyst [Mn2+-azo ligand@APTES-SiO2@Fe3O4] was successfully prepared, characterized and applied for the synthesis of β-alkoxy alcohols using the ring opening of epoxides with alcohols. The catalyst showed a good performance and high turnover frequency. Moreover, the catalyst can be easily retrieved from the reaction mixture and reused without any significant loss in catalytic activity.
Acknowledgements
The authors gratefully acknowledge financial support from the Mahshahr Branch, Islamic Azad University, Iran. Also, we thank Dr. Bijan Nekoueishahraki for helpful discussions and comments on the manuscript.


