1 Introduction
Nitrogen-containing heterocycles are the most abundant and integral scaffolds that exist ubiquitously in a variety of synthetic drugs, pharmaceuticals, bioactive natural products, and agrochemicals [1,2]. Among these heterocycles, quinazolines and their derivatives are important compounds in organic synthesis and the industrial production of pharmaceutical compounds that show various biological activities [3–9] such as anticancer [3–5], antiviral [6], antibacterial [7], and antitubercular [9] ones. The first synthesis of a quinazoline derivative, 2-cyano-3,4-dihydro-4-oxoquinazoline, by reacting cyanogen with anthranilic acid was reported by Griess in 1869. Since then, there has been a spurt at activity to synthesize quinazolines because of their biological importance [10,5a]. A growing importance of quinazolines provides a strong rationale to the development of various synthetic methodologies. Substituted quinazolines have been synthesized by various methods involving several substrates such as 2-aminobenzylamines (I), 2-aminobenzaldehydes and 2-aminobenzoketones (II), or 2-bromobenzylamines (III) (Scheme 1) [11].
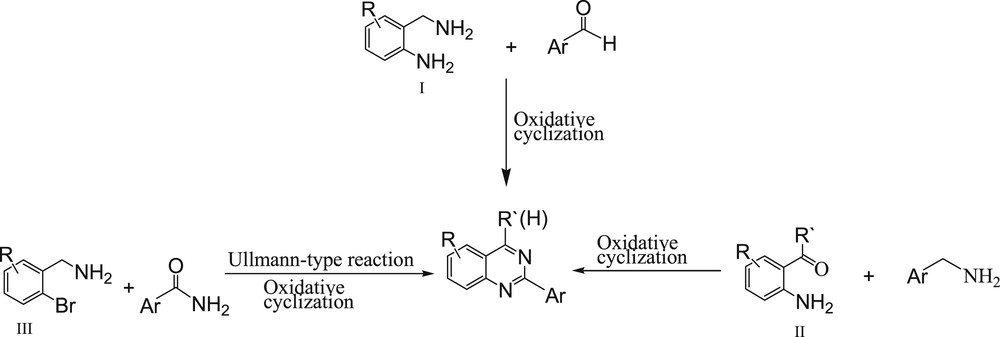
Approaches for the synthesis of quinazolines.
Pyridines also are among the most important heterocycles that are often found in natural products, flavors and fragrances, pharmaceuticals, additives, agrochemicals, dyes, and polymers. They are used as ligands in coordination chemistry and as reagents and building blocks in organic synthesis [12]. One of the most popular methods for preparation of pyridines is oxidation of corresponding 1,4-dihydropyridines, which in turn can be synthesized via the classical Hantzsch 1,4-dihydropyridine synthesis (Scheme 2) [13].
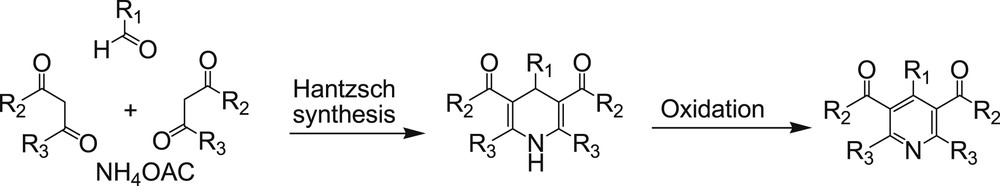
Synthesis and oxidation of Hantzsch 1,4-dihydropyridines.
In view of the importance of quinazolines and pyridines, a large number of stoichiometric or large excess amounts of oxidants and homogenous and heterogeneous catalysts have been reported in the literature for synthesis of 2-substituted quinazolines and Hantzsch pyridines from oxidative cyclocondensation of 2-aminobenzylamines and aldehydes and oxidative aromatization of 1,4-dihydropyridines, respectively. Typical examples such as MnO2 [14], NaClO [15], and 2,3-dichloro-5,6-dicyano-l,4-benzoquinone (DDQ) [16], Ir-catalyzed hydrogen transfer reaction [17], Pt/CeO2 [18], FeCl3 [19], pyridinium chlorochromate [20a], (NH4)2Ce(NO3)6 [20b], MnO2 [20c], NO [20d], HNO3 [20e], Zr(NO3)4 [20f], and 1,2,4-triazolinedione [20g]. However, most of these methods suffer from one or more following drawbacks: hard reaction conditions, poor atom efficiency, utilization of environmentally unfavorable and expensive oxidants and catalysts, tedious procedure for the preparation of homogenous and heterogeneous catalysts, and the formation of large amounts of toxic waste. Therefore, the development of more efficient and green methods is important for synthesis of quinazolines and pyridines. The use of “green oxidants” such as molecular oxygen or air is attractive, because they are readily available, inexpensive, and environmentally benign [21]. Thus, aerobic oxidative synthesis of these valuable compounds in the presence of transition metal catalysts or organocatalysts has been developed [22]. Typical examples include the use of 5 mol% of CuCl/DABCO/TEMPO (DABCO, 1,4-diazabicyclo[2.2.2]octane and TEMPO, 2,2,6,6-tetra-methyl-piperidin-1-yloxy) catalytic system [23], Pt/Ir bimetallic nanoclusters in combination with catechol cocatalysts [24], the assembly of rhodium (Rh) nanoparticles on carbon nanotubes (CNTs) along with redox-active quinone-type cocatalyst [25], 1,10-phenanthroline-5,6-dione associated with different metals (Zn, Fe, or Ru) [26], Fe(ClO4)3/acetic acid [27a], Fe(ClO4)3/N-hydroxyphthalimide [27b], Fe(ClO4)3/triarylaminium radical cation salt [27c], and FeCl3/NaNO2/TEMPO [27d]. Although these catalyst systems could efficiently perform oxidation reactions, these procedures still suffer from some problems such as high reaction temperatures, low catalytic efficiency, tedious catalyst preparation, and the use of toxic organic solvents and toxic transition metals as catalyst, which are essentially challenging to separate from the reaction mixture. Even when it is possible to separate the catalyst, trace amounts of the catalyst are possible to stay in the final product. It is vital to eliminate the catalyst (especially in pharmaceutical industry) because metal contamination is highly regulated. Therefore, it seems that there is still a great interest in developing efficient, eco-friendly, and nonmetallic catalysts for the aerobic oxidative synthesis of quinazolines and Hantzsch pyridines.
One particular stoichiometric organo-oxidant that is capable of performing a wide range of organic transformations is DDQ [28,29]. However, the stoichiometric use of DDQ results in equimolar quantities of the 2,3-dichloro-5,6-dicyano-hydroquinone (DDQH2) byproduct that leads to difficult purification on a large scale. Also, DDQ is relatively expensive ($680/mol) and toxic (LD50 of 82 mg/kg). Consequently, to avoid these disadvantages, the use of a catalytic amount of DDQ in the presence of economically and environmentally benign oxidants that regenerate DDQ from its reduced hydroquinone form has received increasing attention as a possible alternative to the widely used traditional DDQ in organic synthesis. Some of the stoichiometric co-oxidants that have been used for the regeneration of DDQ include FeCl3 (3 equiv) [30], Mn(OAc)3 (3 equiv) [31], and MnO2 (up to 6 equiv) [32]. Although these methods have partly addressed some problems of the stoichiometric DDQ, however these reagents show poor atom efficiency. Green chemistry encourages the design of green processes using O2 or air as the highly atom-economical, environmentally benign, and abundant oxidant and minimizing the generation of hazardous materials. So, it would be ideal if molecular oxygen or air can serve as a terminal oxidant in DDQ-mediated reactions. Nonetheless, molecular oxygen cannot directly oxidize DDQH2 to DDQ under mild conditions [33]. Indeed, a redox compound should be used as the bridge among molecular oxygen and the cycling DDQH2/DDQ. In this context, several research groups have used NO/NO2-based redox cycle systems in combination with DDQ to catalyze the aerobic oxidation of organic compounds, including oxidation of alcohols [34], oxidative cleavage of benzylic ethers [35], dehydrogenation of saturated CC bonds [36], oxidative CC coupling reactions, and amination of arenes [37]. Very recently, Fe(NO3)3 has been used as a cocatalyst with catalytic DDQ in aerobic oxidation of alcohols [38]. Although these methods presented interesting breakthrough in the field of aerobic oxidation reactions in the presence of catalytic amount of DDQ, the development of an efficient and eco-friendly cocatalyst in combination with DDQ to catalyze the aerobic oxidation of organic compounds under mild conditions is still highly desired.
Laccases (p-benzenediol: oxygen oxidoreductase [E.C. 1.10.3.2]) are extracellular enzymes that contain four copper centers per protein molecule and catalyze the oxidation of electron-rich aromatic substrates, usually phenols or aromatic amines [39]. The unique properties of laccases such as mild reaction conditions and substrate selectivity make them attractive for use in chemical synthesis [40]. Furthermore, the redox potential of laccases can be extended via natural mediators such as 3-hydroxyanthranilic acid and 4-hydroxybenzoic acid as well as by artificial mediators including TEMPO, 1-hydroxybenzotriazole, and 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) [41]. This allows the oxidation of substrates with higher redox potentials. Meanwhile, laccases and laccase/mediator systems have been applied for the oxidation of a number of functional groups [42–44]. To the best of our knowledge, there has been no report on the use of laccase/DDQ as a bioinspired cooperative catalyst system in the transition metal–free and halogen-free aerobic oxidation of organic compounds (Scheme 3).

Proposed DDQ-mediated aerobic oxidation reactions.
In continuation to our systematic study about application of laccase and laccase mediator system in organic synthesis [45], herein we present for the first time the nonmetallic, simple, and efficient methods for the synthesis of 2-substituted quinazolines and Hantzsch pyridines via aerobic oxidative in the presence of the laccase/DDQ catalyst system, using O2 or air as an oxidant and a phosphate buffer solution as a solvent.
2 Experimental section
2.1 Materials and instruments
Laccase (E.C. 1.10.3.2) from Trametes versicolor was purchased from Sigma–Aldrich (St. Louis, MO, USA). Sodium acetate buffer (100 mM) at pH 5 was used for preparing solutions for the activity assay. All chemicals and solvents were of analytical grade and used without further purification. 1H NMR spectra were recorded using a 250 MHz Bruker NMR spectrometer. Melting points were measured on a Barnstead Electrothermal IA 9100 and are uncorrected.
2.2 Enzyme activity assays
The activity of commercial enzyme laccase from T. versicolor was determined spectrophotometrically by monitoring the oxidation of 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, ε = 36,000 M−1 cm−1). The reaction mixture included 5 mM of ABTS (100 μL) in 100 mM of acetate buffer (pH 5.0) and laccase enzyme. The change in absorption was observed via UV–vis spectroscopy at 420 nm for 5 min at room temperature [46]. One unit was defined as the amount of enzyme that oxidizes 1 μmol of ABTS per minute. The activity of laccase enzyme batch applied in this investigation was evaluated at 0.89 U/mg.
2.3 General procedure for the aerobic oxidative synthesis of 2-substituted quinazolines by laccase/DDQ catalyst system
A solution of 2-aminobenzylamine (1 mmol) and aldehydes (1 mmol) in 5 mL of CH3CN was stirred at room temperature until the cyclocondensation was completed (about 3–5 h). After complete conversion of 2-aminobenzylamine to tetrahydroquinazoline (TLC), the solvent was concentrated to 0.5 mL and DDQ (45.4 mg, 0.2 mmol), and sodium phosphate buffer solution (NaPBS) (0.1 M, pH 4.5, 12.5 mL) and laccase from T. versicolor (100 U, 89 mg) were added. Then, the mixture was stirred at 45 °C under molecular oxygen (balloon) or air for the time given in Table 1. After completion of the reaction (monitored by TLC), the mixture was extracted by EtOAc (3 × 10 mL) and dried over anhydrous Na2SO4. The crude product obtained after the removal of a solvent under vacuum was purified by chromatography on silica gel (n-hexane/ethyl acetate, 3/1). All of the products were identified and characterized by comparing their spectral data (1H NMR) and physical properties (melting point) with those of authentic samples (see Supplementary data).
Laccase/DDQ-catalyzed aerobic oxidative synthesis of pyridine derivatives.a
| Entry | Substrates | Product | Time (h) | Yield (air)b (%) |
| 1 | Image 28 | Image 40 | 7 | 98 (87) |
| 2 | Image 29 | Image 41 | 10 | 96 (84) |
| 3 | Image 30 | Image 42 | 8 | 94 (80) |
| 4 | Image 31 | Image 43 | 6 | 97 (85) |
| 5 | Image 32 | Image 44 | 7 | 93 (79) |
| 6 | Image 33 | Image 45 | 12 | 95 (81) |
| 7 | Image 34 | Image 46 | 14 | 90 (78) |
| 8 | Image 35 | Image 47 | 12 | 98 (89) |
| 9 | Image 36 | Image 48 | 12 | 97 (86) |
| 10 | Image 37 | Image 49 | 16 | 98 (88) |
| 11 | Image 38 | Image 50 | 11 | 96 (83) |
| 12 | Image 39 | Image 51 | 14 | 90 (76) |
a General procedure: 1,4-Dihydropyridine (1 mmol), laccase (100 U), DDQ (20 mol%), O2 (balloon), NaPBS (12.5 mL), rt.
b Isolated yield under air conditions is shown in parentheses.
2.4 General procedure for the aerobic oxidative synthesis of pyridines by laccase/DDQ catalyst system
A 25 mL round-bottomed flask equipped with a magnetic stirrer bar was charged with 1,4-dihydropyridine (1 mmol), DDQ (45.4 mg, 0.2 mmol), and acetonitrile (0.5 mL). NaPBS (0.1 M, pH 4.5, 12.5 mL) and laccase from T. versicolor (100 U, 89 mg) were added and the reaction mixture was stirred under molecular oxygen (balloon) or air at room temperature for the time given in Table 2. After completion of the reaction (monitored by TLC), the mixture was extracted by ethyl acetate (3 × 10 mL) and dried over anhydrous Na2SO4. The crude product obtained after the removal of a solvent under vacuum was purified by chromatography on silica gel (n-hexane/ethyl acetate, 3/1). All of the products were identified and characterized by comparison of their spectral data (1H NMR) and physical properties (melting point) with those of authentic samples (see Supplementary data).
Optimization of reaction conditions for aerobic oxidation of diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate.a
| Entry | Laccase (U) | Solvent | Temperature (oC) | DDQ (mol %) | GC yield (%) |
| 1 | 100 | CH3CN/NaPBS | rt | – | – |
| 2 | 100 | CH3CN/NaPBS | rt | 5 | 70 |
| 3 | 100 | CH3CN/NaPBS | rt | 10 | 80 |
| 4 | 100 | CH3CN/NaPBS | rt | 20 | 100 |
| 5 | 50 | CH3CN/NaPBS | rt | 20 | 70 |
| 6 | – | CH3CN/NaPBS | rt | 20 | 40 |
| 7 | 100 | CH3OH/NaPBS | rt | 20 | 90 |
| 8 | 100 | NaPBS buffer | rt | 20 | 60 |
| 9 | 100 | H2O | rt | 20 | 40 |
| 10 | 100 | CH3CN | rt | 20 | – |
| 11 | 100 | THF | rt | 20 | – |
| 12 | 100 | DMF | rt | 20 | – |
| 13 | 100 | DMSO | rt | 20 | – |
| 14 | 100 | CH3CN/NaPBS | 60 | 20 | 40 |
| 15b | 100 | CH3CN/NaPBS | 45 | 20 | 100 |
| 16c | 100 | CH3CN/NaPBS | rt | 20 | 90 |
a Reaction conditions: 1,4-Dihydropyridine (1 mmol), laccase (100 U), O2 (balloon), and solvent (13 mL).
b Reaction time was 3 h.
c The reaction was carried out under air conditions.
2.5 Selected spectra data
2.5.1 2-Phenyl quinazoline
Pale yellow solid; mp 97–98 °C: 95% yield (196 mg, 0.95 mmol) [23] (Table 1, entry 1).
1H NMR (250 MHz, DMSO-d6): δ = 9.70 (s, 1H), 8.54–8.57 (m, 2H), 8.16 (d, J = 8.0 Hz, 1H), 7.99–8.04 (m, 2H), 7.70–7.76 (m, 1H), 7.55–7.56 (m, 1H).
2.5.2 2-(4-Methoxylphenyl) quinazoline
White solid; mp 78–80 °C: 96% yield (226 mg, 0.96 mmol) [23] (Table 1, entry 2).
1H NMR (250 MHz, DMSO-d6): δ = 9.61 (s, 1H), 8.49 (d, J = 8.0 Hz, 2H), 8.10 (d, J = 7.5 Hz, 1H), 7.98 (br s, 2H), 7.66–7.67 (m, 1H), 7.08 (d, J = 8.0 Hz, 2H), 3.82 (s, 3H).
2.5.3 2-(3-Methoxyphenyl) quinazoline
Light yellow solid; mp 91–93 °C: 94% yield (222 mg, 0.94 mmol) [26] (Table 1, entry 3).
1H NMR (250 MHz, DMSO-d6): δ = 9.69 (s, 1H), 8.05–8.17 (m, 5H), 7.73–7.76 (m, 1H), 7.47 (t, J = 8.0 Hz, 1H), 7.11–7.13 (m, 1H), 3.86 (s, 3H).
2.5.4 2-(3-Fluorophenyl) quinazoline
Gray solid; mp 89–90 °C: 83% yield (186 mg, 0.83 mmol) [47] (Table 1, entry 7).
1H NMR (250 MHz, DMSO-d6): δ = 9.70 (s, 1H), 8.39 (d, J = 8.0 Hz, 1H), 8.15–8.25 (m, 2H), 8.03–8.05 (m, 2H), 7.72–7.78 (m, 1H), 7.60 (dd, J = 21.7, 6.2 Hz, 1H), 7.36–7.42 (m, 1H).
2.5.5 2-(3-Chlorophenyl) quinazoline
Pale yellow solid; mp 148–150 °C: 93% yield (223 mg, 0.93 mmol) [23] (Table 1, entry 8).
1H NMR (250 MHz, DMSO-d6): δ = 9.70 (s, 1H), 8.51 (br s, 2H), 8.17 (d, J = 8.0 Hz, 1H), 8.0–8.06 (m, 2H), 7.72–7.78 (m, 1H), 7.60 (br s, 2H).
2.5.6 2-(3-Bromophenyl)quinazoline
Yellow solid; mp 153–155 °C: 91% yield (259 mg, 0.91 mmol) [23] (Table 1, entry 9).
1H NMR (250 MHz, DMSO-d6): δ = 9.71(s, 1H), 8.67 (s, 1H), 8.53 (d, J = 7.5 Hz, 1H), 8.17 (d, J = 8.0 Hz, 1H), 8.01–8.06 (m, 2H), 7.73–7.76 (m, 2H), 7.53 (t, J = 8.0 Hz, 1H).
2.5.7 Diethyl 2,6-dimethyl-4-phenyl-3,5-pyridinedicarboxylate
Pale yellow solid; mp 60–61 °C: 98% yield (320 mg, 0.98 mmol) [48] (Table 2, entry 1).
1H NMR (250 MHz, CDCl3): δ = 7.25–7.35 (m, 5H), 3.98 (q, J = 6.7 Hz, 4H), 2.59 (s, 6H), 0.88 (t, J = 6.7 Hz, 6H).
2.5.8 Diethyl 2,6-dimethyl-4-(p-tolyl)-3,5-pyridinedicarboxylate
Pale yellow solid; mp 72–74 °C: 96% yield (327 mg, 0.96 mmol) [48] (Table 2, entry 2).
1H NMR (250 MHz, CDCl3): δ = 7.14–7.26 (m, 4H), 4.02 (q, J = 7.0 Hz, 4H), 2.58 (s, 6H), 2.36 (s, 3H), 0.95 (t, J = 7.0 Hz, 6H).
2.5.9 Diethyl 2,6-dimethyl-4-(4-methoxyphenyl)-3,5-pyridinedicarboxylate
Colorless solid; mp 49–51 °C: 94% yield (335 mg, 0.94 mmol) [48] (Table 2, entry 3).
1H NMR (250 MHz, CDCl3): δ = 7.17 (d, J = 8.2 Hz, 2H), 6.8 (d, J = 8.2 Hz, 2H), 4.03 (q, J = 7 Hz, 4H), 3.80 (s, 3H), 2.56 (s, 6H), 0.96 (t, J = 7 Hz, 6H).
2.5.10 Diethyl 2,6-dimethyl-4-(3,4,5-trimethoxyphenyl)-3,5-pyridinedicarboxylate [49]
White solid; mp 105–107 °C: 97% yield (404 mg, 0.97 mmol) (Table 2, entry 4).
1H NMR (250 MHz, CDCl3): δ = 6.49 (s, 2H), 4.06 (q, J = 7.0 Hz, 4H), 3.84 (s, 3H), 3.80 (s, 6H), 2.58 (s, 6H), 0.98 (t, J = 7.0 Hz, 6H).
2.5.11 Diethyl 2,6-dimethyl-4-n-butyl-3,5-pyridinedicarboxylate
Oil; 93% yield (285 mg, 0.93 mmol) [48] (Table 2, entry 5).
1H NMR (250 MHz, CDCl3): δ = 4.39 (q, J = 7.0 Hz, 4H), 2.53–2.56 (m, 2H), 2.49 (s, 6H), 1.51–1.60 (m, 4H), 1.37 (t, J = 7.0 Hz, 6H), 0.91 (t, J = 7.0 Hz, 3H).
2.5.12 Diethyl 2,6-dimethyl-4-(3-chlorophenyl)-3,5-pyridinedicarboxylate
Pale yellow solid; mp 42–44 °C: 98% yield (354 mg, 0.98 mmol) [50] (Table 2, entry 8).
1H NMR (250 MHz, CDCl3): δ = 7.25–7.34 (m, 3H), 7.12–7.15 (m, 1H), 4.05 (q, 7.0 Hz, 4H), 2.60 (s, 6H), 0.97 (t, J = 7.0 Hz, 6H).
2.5.13 Diethyl 2,6-dimethyl-4-(2-fluorophenyl)-3,5-pyridinedicarboxylate [51]
Oil; 97% yield (334 mg, 0.97 mmol) (Table 2, entry 9).
1H NMR (250 MHz, CDCl3): δ = 7.25–7.34 (m, 1H), 6.97–7.10 (m, 3H), 4.03 (q, J = 7.0 Hz, 4H), 2.59 (s, 6H), 0.95 (t, J = 7.0 Hz, 6H).
2.5.14 Diethyl 2,6-dimethyl-4-(4-nitrophenyl)-3,5-pyridinedicarboxylate
Pale yellow solid; mp 115–117 °C: 98% yield (364 mg, 0.98 mmol) [48] (Table 2, entry 10).
1H NMR (250 MHz, CDCl3): δ = 8.24 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 4.02 (q, J = 7.0 Hz, 4H), 2.62 (s, 6H), 0.97 (t, J = 7.0 Hz, 6H).
2.5.15 Diethyl-2,6-dimethyl-4-cinamyl-3,5-pyridinedicarboxylate
Pale yellow solid; mp 164–165 °C: 90% yield (318 mg, 0.90 mmol) [48] (Table 2, entry 12).
1H NMR (250 MHz, CDCl3): δ = 7.26–7.41 (m, 5H), 7.10 (d, J = 16.5 Hz, 1H), 6.79 (d, J = 16.5 Hz, 1H), 4.32 (q, J = 7.0 Hz, 4H), 2.56 (s, 6H), 1.27 (t, J = 7.0 Hz, 6H).
3 Results and discussion
To optimize the reaction conditions, the dehydrogenation of 2-phenyl tetrahydroquinazoline as the model reaction was investigated. Initially, the reaction was carried out in the presence of laccase (100 U) and O2 as the oxidant in a CH3CN/NaPBS mixture (1:25) as the solvent at 45 °C. Very low conversions of starting materials were observed, by performing the reactions in the absence of DDQ (Table 3, entry 1). When DDQ (5 mol %) was added as a mediator in the reaction, the product was obtained in moderate yield (Table 3, entry 2). These results demonstrated that DDQ is necessary for an efficient reaction. By increasing the amount of DDQ to 10 mol% the yield slightly improved (Table 3, entry 3). The excellent result was obtained when 20 mol% of DDQ was used (Table 3, entry 4). Subsequently, the effect of laccase concentration on the reaction was explored. When the amount of laccase was reduced to 50 U, the yield dropped to 70% (Table 3, entry 5). A blank reaction was carried out to evaluate the catalytic efficiency of laccase where only 30% of desired product was obtained (Table 3, entry 6). The optimal enzyme concentration was found to be 100 U (entry 4). So, it was selected as a standard concentration in all further reactions. Among various solvents screened, such as CH3CN/NaPBS (pH 4.5, 0.1 M), CH3OH/NaPBS (pH 4.5, 0.1 M), NaPBS buffer, and H2O, CH3CN/NaPBS (pH 4.5, 0.1 M) gave the best result (Table 3, entries 4 and 7–9). In organic solvents, such as CH3CN, THF, DMF, and DMSO the reactions did not occur, probably because of laccase denaturation (Table 3, entries 10–13). The effect of temperature, both high and low, on the reaction yield was also tested. It was found that by increasing the reaction temperature up to 60 °C, the yield was dropped because of enzyme deactivation [52] (Table 3, entry 14). At room temperature, the desired product was obtained in moderate yield (Table 3, entry 15). Using air as an oxidant instead of molecular oxygen in the reaction gave 90% conversion of 2-phenyl tetrahydroquinazoline (Table 3, entry 16).
Laccase/DDQ-catalyzed aerobic oxidative synthesis of quinazoline derivatives.a
| Entry | RCHO | Product | Time (h) | Isolated yield (air)b (%) |
| 1 | Image 4 | Image 15 | 22 | 95 (87) |
| 2 | Image 5 | Image 16 | 20 | 96 (84) |
| 3 | Image 6 | Image 17 | 24 | 94 (86) |
| 4 | Image 7 | Image 18 | 26 | 93 (85) |
| 5 | Image 8 | Image 19 | 20 | 93 (82) |
| 6c | Image 9 | Image 20 | 24 | 91 (80) |
| 7 | Image 10 | Image 21 | 24 | 83 (77) |
| 8 | Image 11 | Image 22 | 22 | 93 (84) |
| 9 | Image 12 | Image 23 | 24 | 91 (80) |
| 10 | Image 13 | Image 24 | 24 | 72 (64) |
| 11 | Image 14 | Image 25 | 24 | 87 (79) |
a General procedure: Aldehyde (1 mmol), 2-aminobenzylamine (1 mmol), laccase (100 U), DDQ (20 mol%), O2 (balloon), NaPBS (12.5 mL), CH3CN (0.5 mL), 45 °C.
b Isolated yield under air conditions is shown in parentheses.
c Conditions: Aldehyde (1 mmol), 2-aminobenzylamine (2 mmol), laccase (200 U), DDQ (40 mol%), O2 (balloon), NaPBS (25 mL), CH3CN (1 mL), 45 °C.
With having the optimized catalytic system laccase/DDQ in hand, the general applicability of this method was further examined for the synthesis of 2-substituted quinazolines from the oxidative cyclocondensation of 2-aminobenzylamine and structurally various aldehydes. Under the optimized reaction conditions (Table 3, entries 4 and 16), the expected products were obtained in excellent yields and the results are shown in Table 1. Different substituents, including both electron-poor (fluoro, chloro, and bromo) and electron-rich (methyl, methoxy) groups, at various positions of the benzaldehyde ring would be compatible by the present oxidative system and gave the desired products in high yields (Table 1, entries 2–11). Notably, terephthaldehyde as a bifunctional aromatic aldehyde was satisfactorily subjected to oxidative cyclocondensation as well (Table 1, entry 6).
Having successfully achieved the aerobic oxidative synthesis of 2-substituted quinazolines, we expanded the application of laccase/DDQ catalytic system for aerobic oxidation of Hantzsch 1,4-dihydropyridines to pyridines. 1,4-Dihydropyridines were synthesized according to the reported procedure [53]. The effect of amount of laccase and DDQ, solvent, and temperature on the oxidation reaction of diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate to diethyl 2,6-dimethyl-4-phenyl-3,5-pyridinedicarboxylate as a model reaction was investigated (Table 4). Laccase (100 U) and 20 mol% of DDQ under O2 or air in NaPBS (0.1 M, pH 4.5)/CH3CN (4%) mixture at room temperature was found to be ideal for complete conversion of 1,4-dihydropyridines to the corresponding pyridines (Table 4, entry 4).
Optimization of reaction conditions for aerobic oxidation of 2-phenyl tetrahydroquinazoline.a
| Entry | Laccase (U) | Solvent | Temperature (°C) | DDQ (mol %) | GC yield (%) |
| 1 | 100 | CH3CN/NaPBS | 45 | – | – |
| 2 | 100 | CH3CN/NaPBS | 45 | 5 | 60 |
| 3 | 100 | CH3CN/NaPBS | 45 | 10 | 70 |
| 4 | 100 | CH3CN/NaPBS | 45 | 20 | 100 |
| 5 | 50 | CH3CN/NaPBS | 45 | 20 | 70 |
| 6 | – | CH3CN/NaPBS | 45 | 20 | 30 |
| 7 | 100 | CH3OH/NaPBS | 45 | 20 | 90 |
| 8 | 100 | NaPBS buffer | 45 | 20 | 50 |
| 9 | 100 | H2O | 45 | 20 | 40 |
| 10 | 100 | CH3CN | 45 | 20 | – |
| 11 | 100 | THF | 45 | 20 | – |
| 12 | 100 | DMF | 45 | 20 | – |
| 13 | 100 | DMSO | 45 | 20 | – |
| 14 | 100 | CH3CN/NaPBS | 60 | 20 | 40 |
| 15 | 100 | CH3CN/NaPBS | rt | 20 | 50 |
| 16b | 100 | CH3CN/NaPBS | 45 | 20 | 90 |
a Reaction conditions: 2-Phenyl tetraquinazoline (1 mmol), laccase (100 U), O2 (balloon), and solvent (13 mL).
b The reaction was carried out under air conditions.
To generalize the scope of the reaction, a series of structurally diverse Hantzsch 1,4-dihydropyridines were subjected to aerobic oxidation under the optimized reaction conditions, and the results are presented in Table 2.
To investigate the feasibility of applying this chemoenzymatic synthetic method on a preparative scale, the aerobic oxidation of diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate was carried out on a 5 mmol scale of starting materials. We have found the scale up of reaction gave limited success and shows the lower substrate conversion (60%) [54].
Although the exact mechanisms of the reactions are not clear at this stage, however according to previous reports on the application of DDQ in organic reactions and aerobic oxidation of hydroquinone to quinone by laccase [45,55–57], the proposed mechanisms for the aerobic oxidation of 2-substituted tetrahydroquinazolines and 1,4-dihydropyridines are presented in Schemes 4 and 5, respectively.

Radical path mechanism for the aerobic oxidation of 2-substituted tetrahydroquinazolines (a) and 1,4-dihydropyridine (b) in the presence of laccase/DDQ catalyst system.

Electron transfer path or anomeric-based oxidation mechanism for the aerobic oxidation of 2-substituted tetrahydroquinazolines (a) and 1,4-dihydropyridine (b) in the presence of the laccase/DDQ catalyst system.
One explanation is that a single electron transfer from the 2-substituted tetrahydroquinazolines or 1,4-dihydropyridines to DDQ generates a radical cation (intermediates A and B in Scheme 4) and a DDQ radical anion. The radical oxygen of the DDQ radical anion abstracts an α-H-atom and then the anionic oxygen of DDQ radical anion abstracts proton from nitrogen to form target products (2-substituted quinazolines and pyridines) and DDQH2. The reduced DDQH2 (hydroquinone type compound) is subsequently regenerated by laccase leading to DDQ and reduced form of laccase. Finally, laccase can be reoxidized by molecular oxygen, thus completing the catalytic cycle (Scheme 4a and b).
Another explanation for these processes is that oxidation of the substrate occurs by hydride transfer from the C2 and C4 position on 2-substituted tetrahydroquinazolines and C4 position on 1,4-dihydropyridines to the carbonyl oxygen atom of DDQ (transition states C and E), thereby forming ion-pairs (intermediates D and F) [56]. It should be mentioned that these transformations may proceedvia anomeric-based oxidation [20g]. Deprotonation of the substrate by DDQH− can afford the corresponding dehydrogenated product. The reduced DDQH2 is oxidized by laccase leading to DDQ and reduced form of laccase. Finally, laccase can be reoxidized by molecular oxygen, thus completing the catalytic cycle (Scheme 5).
To illustrate the efficiency of laccase/DDQ catalyst system, we compared our results for dehydrogenation of 2-phenyl tetrahydroquinazoline and diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate with the best of the well-known data from the literature (Tables 5 and 6). As shown in Tables 5 and 6, the previously reported procedures suffer from one or more disadvantages such as the use of stoichiometric oxidant [14,15,20c,20d,20f,58,59], toxic and expensive transition metal catalysts or reagent [14,17,19,20c,20f,23–26,27a,27d], special efforts for the preparation of catalyst [17,24,25], toxic and volatile solvents [14–19,20c,20d,23–25,27b–27d,56,60], elevated reaction temperatures [14,17,19,23,20c,27b,27c,44], and low yield [14,17,19,27c,44].
Comparison of the laccase/DDQ catalyst system with other reagent or catalysts for oxidation of 2-phenyl tetrahydroquinazoline to 2-phenyl quinazoline.
| Entry | Reagent or catalyst | Reaction conditions | Time (h) | Isolated yield (%) | Reference |
| 1 | MnO2 (4 mmol) | CHCl3, reflux | 12 | 70 | [14] |
| 2 | NaOCl (3 mmol) | MeOH, rt | 5 | 98 | [15] |
| 3 | [CpIrCl2]2 (2.5 mol%), styrene (4 equiv) | N2, xylene, reflux | 24 | 66 | [17] |
| 4 | Pt/CeO2 (1 mol %) | Mesitylene, N2, reflux | 30 | 85 | [18] |
| 5 | FeCl3 (2 mol%), TBHP (1.5 mol%) | DMSO, N2, 60 °C | 6 | 70 | [19] |
| 6 | IBX (2 equiv) | CH3CN, rt | 6 | 88 | [57] |
| 7 | CuCl (5 mol%)/DABCO (10 mol%)/TEMPO (5 mol%) | CH3CN, O2, 80 °C | 6 | 95 | [23] |
| 8 | PI/CB-Pt/Ir (0.5 mol%), TTSBI (3.5 mol%), K2CO3 (0.5 equiv) | CHCl3: H2O (9:1, 0.5 M), O2, 35 °C | 16 | 95 | [24] |
| 9 | RhCNT (1 mol%), TBC (10 mol%) | CHCl3/H2O (3:1), rt | 21 | 94 | [25] |
| 10 | phd (5 mol%), ZnI2 (2.5 mol%), PPTS (15 mol%) | CH3CN, O2, rt | 24 | 84 | [26] |
| 11 | MgI2 (5 mol%) | EtOAC, visible light, O2, rt | 8 | 87 | [59] |
| 12 | Laccase (100 U)/DDQ (20 mol%) | NaPBS/CH3CN, O2 or air, 45 °C | 22 | 95 | This work |
Comparison of laccase/DDQ catalyst system, with other reagent or catalysts for oxidation of diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate to diethyl 2,6-dimethyl-4-phenyl-3,5-pyridinedicarboxylate.
| Entry | Reagent or catalyst | Reaction conditions | Time | Isolated yield (%) | Reference |
| 1 | MnO2 | CH2Cl2, MW 100 °C | 1 min | 99 | [20c] |
| 2 | NO (20 equiv) | Benzene, under Ar atmosphere | 3 h | 96 | [20d] |
| 3 | Zr(NO3)4 (1 equiv) | AcOH, rt | 15 min | 96 | [20f] |
| 4 | DDQ (0.5 mmol) | THF, rt | 1 h | 90 | [59] |
| 5 | Fe(ClO4)3 (2 mol%) | HOAC, rt | 1.5 h | 93 | [27a] |
| 6 | NHPI (20 mol%) | CH3CN, O2, reflux | 4 h | 99 | [27b] |
| 7 | TBPA+˙ | CH3CN, air, 60 °C | 18 h | 78 | [27c] |
| 8 | FeCl3 (0.05 mmol)/NaNO2 (0.05 mmol)/TEMPO (0.05 mmol) | CH3CN, CH3COOH, O2, rt | 30 min | 97 | [27d] |
| 9 | Laccase (168 U)/ABTS (10 mol%) | Acetate buffer/MeOH, air, 50 °C | 9 h | 80 | [44] |
| 10 | Laccase (100 U)/DDQ (20 mol%) | NaPBS/CH3CN, O2 or air, rt | 7 h | 98 | This work |
4 Conclusion
We have developed laccase/DDQ as a simple, efficient, and environmentally friendly catalyst system for the aerobic oxidative synthesis of structurally diverse 2-substituted quinazolines and Hantzsch pyridines in good to high yields under mild reaction conditions. These green procedures represent various attractive characteristics, such as the use of laccase/DDQ as the robust catalyst system, O2 or air as the greenest, inexpensive and most abundant oxidant, and phosphate buffer as a green medium. In addition, the present methods are superior to other currently available ones because they are free from any toxic halide and transition metal cocatalysts.
Acknowledgments
We gratefully acknowledge the financial support provided to this research by the University of Mohaghegh Ardabili and the University of Kurdistan.


