1. Introduction
Pathological deposits are very common in the human body and accurate characterization is of major importance for medical care. The research described here was performed in close collaboration with several hospitals [1, 2, 3, 4]. Pathological calcifications and abnormal deposits more generally, such as in tattoos [5] or sarcoidosis [6, 7], may be intimately linked with major health problems such as cancer [8, 9, 10, 11, 12, 13], infection [14, 15, 16, 17, 18, 19], diabetes [20, 21, 22], or genetic disorders [23, 24, 25, 26]. The various reviews published from either a medical [27, 28, 29, 30, 31] or a physicochemical point of view [1, 2, 3, 4, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38] have clearly underlined that characterizing deposits, of endogenous and exogenous origins, in the human body are crucial to not only assist diagnosis but also to optimise and develop specific therapy regimens.
More precisely, recent literature and the results we have gathered on different human tissues, including bone [39, 40, 41], skin [5, 6, 42, 43], thyroid [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46], prostate [8, 47], kidney [42, 43, 44, 45, 46, 47, 48, 49, 50, 51], cartilage [52, 53, 54, 55], breast [9, 56], salivary glands [57], and pancreas [58], showed that characterization by staining is often of limited utility. A more comprehensive approach has to encompass a wide range of techniques able to describe pathological calcifications at different scales. They can be classified into different families; techniques able to define elemental compositions such as X-ray fluorescence (XRF) [59, 60] or energy dispersive X-ray spectroscopies (EDS) [61, 62], those which describe molecular composition such as Fourier transform infrared (FTIR) [63, 64] or Raman spectroscopies [65, 66, 67, 68] as well as scattering techniques [69, 70, 71, 72]. Imaging techniques such as scanning [61, 62] and transmission electron microscopy (SEM and TEM) [73, 74, 75, 76] or micro computed tomographic imaging [77, 78, 79, 80] are also of prime importance. Finally, it is possible to precisely define the electronic state of some elements through X-ray photoelectron spectroscopy (XPS) [81, 82, 83] and/or their local atomic level environment using X-ray absorption spectroscopy (XAS) [84, 85, 86], electron energy-loss spectroscopy (EELS) [76, 87, 88] or nuclear magnetic resonance (NMR) [57, 89, 90, 91].
The micrometer to nanometer scale information about abnormal deposits that these techniques yield allows an accurate description of the processes underlying the pathology, by defining morphology, elemental composition, and molecular and structural aspects. Some of these techniques can be used in vivo on abnormal deposits in human tissues while some can only be used on biopsy material. The application of all of these complementary techniques in the specific characterisation of abnormal human deposits is detailed in the present paper.
2. Defining an analytical procedure
We will consider the analysis procedure in four steps (Figure 1). The first is initial characterization directly on the human body using a portable analytical device at the physician’s surgery or at the injury location depending on severity. The next would be in a hospital in which commercial analytical apparatus is available. The third step involves characterization of biopsies in a physical laboratory performing fundamental analytical research. We conclude by describing experiments requiring large scale instrumentation (often shared with other national and international users) such as synchrotron facilities. Such “photon factories” produce well-collimated and high intensity photon synchrotron radiation beams leading to better spatial resolution and/or higher sensitivity.
Various steps of the experimental approach proposed for structural and chemical investigations of pathological calcifications.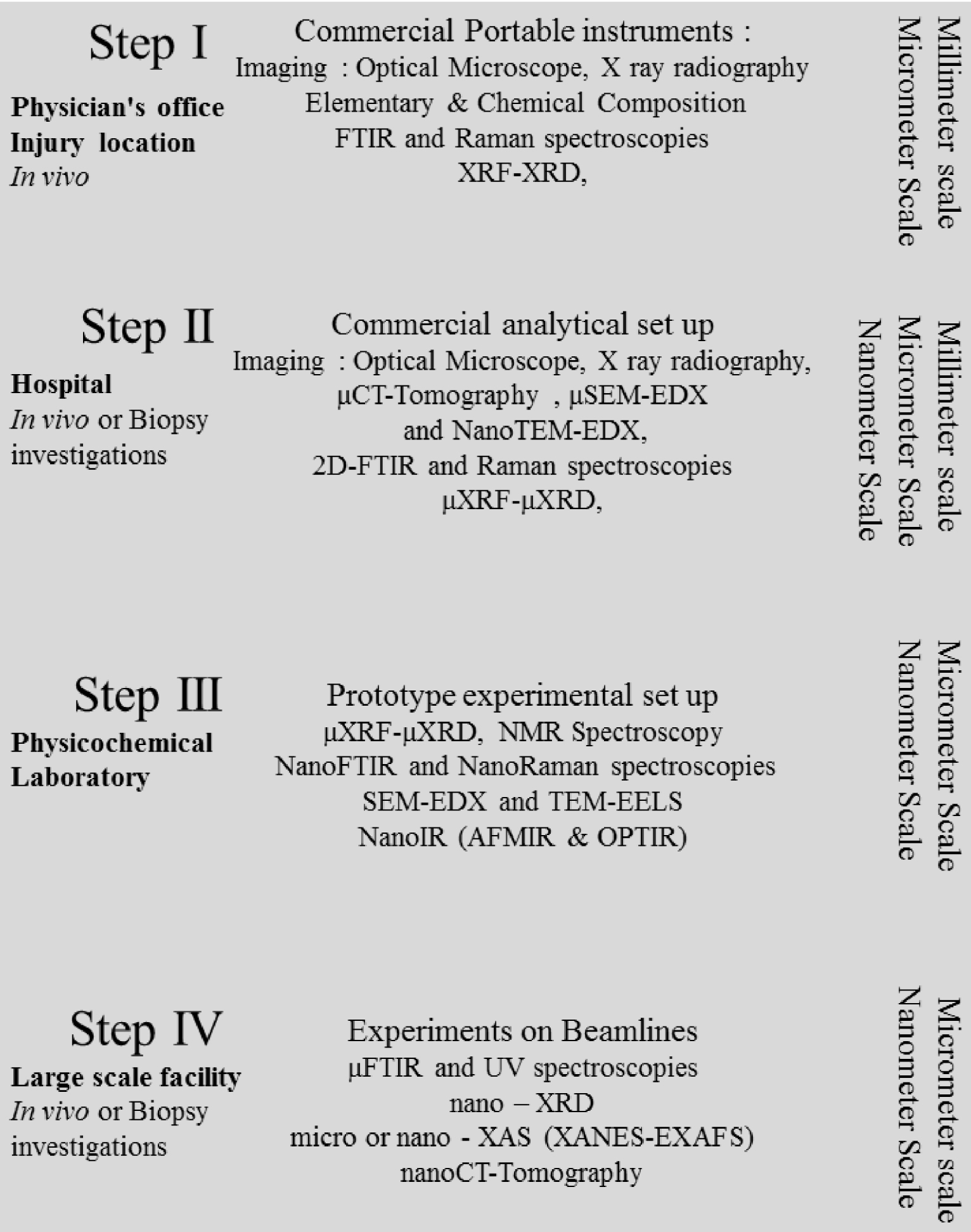
2.1. In vivo analysis at the physician’s surgery or place of injury
The in vivo or in situ (for forensic medicine) step takes account of the fact that different easy-to-implement experimental setups have been developed over the last decades (Figure 2). These include portable radiography apparatus [96], which can define an area of interest for extraction of biological samples if possible and necessary for subsequent characterization.
In vivo portable devices for (a) Radiography [92], (b) XRF [93], (c) FTIR [94] and (d) Raman [95] spectroscopies.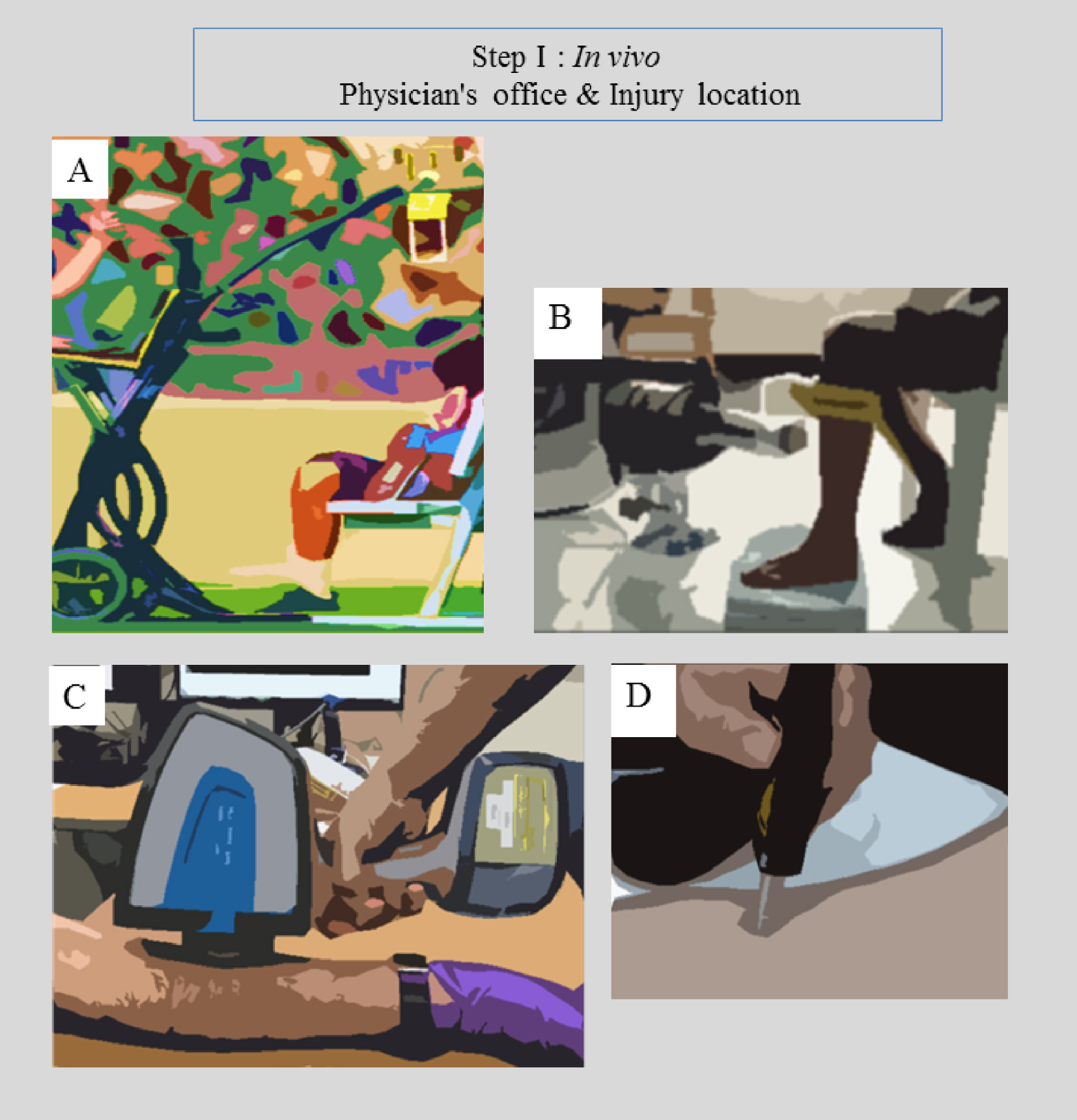
XRF (for elemental analysis and quantification) [97, 98], FTIR [99, 100, 101, 102] or Raman [103, 104, 105] spectroscopies (to determine the nature and amount of chemical compounds present in the sample) constitute at least three characterization techniques which can be applied in vivo. It is worth mentioning that biological fluids (or actual tissue in the case of accident trauma) can also be analysed, in which case, two other techniques namely X-ray Diffraction (XRD, to determine the nature and quantity of any crystalline phases present in the sample) and tabletop SEM (for submicrometer scale surface imaging) can also be applied.
More precisely, XRD can be used to determine the crystallographic structure of unknown deposits [106, 107], to identify the different crystalline phases and their composition, concentration, and average crystal dimensions [72, 108]. “Tabletop” SEM is also available for observing the micrometer scale surface topology of the sample [109] and ultimately determine particle elemental composition by EDS spectroscopy [72].
2.2. Analysis at the hospital on the patient (biopsy)
The second step in a concerted analysis would be performed in a hospital laboratory where commercial physicochemical setups can be used (Figure 3). Investigations in vivo as well as on biopsies are possible. Biopsy procedures are usually performed at the hospital under and/or after radiological examination in the case of breast [110] or kidney [111]. A commercial in-lab setup provides analytical data superior to that produced by portable apparatus (better spatial resolution and sensitivity); the three techniques considered above, namely SEM, XRF and XRD can be performed directly on the biopsy sample (i.e. before paraffin embedding). Note that at Tenon Hospital nanometer scale biopsy characterization by TEM is also possible (Figure 3E).
Tabletop commercial instruments (A) Classical FTIR experimental set up, (B) Raman experimental set up (implemented at the synchrotron SOLEIL), (C) μFTIR microscope implemented at the Service des Explorations Fonctionnelles at the Tenon Hospital, (D) Tabletop SEM (from https://www.hitachi-hightech), (E) TEM experimental set up implemented at the Tenon Hospital.
Embedding the biopsy in a paraffin block allows the sample to be preserved and manipulated simply and safely. It also offers the opportunity to collect a set of data yielding elemental composition (through μXRF or μEDX experiments) or chemical mapping (μFTIR, μRaman or μXRD experiments), from the same sample. Note that for μXRF and μXRD experiments, it is better to use the paraffin block itself, while for μFTIR or μRaman experiments sectioning of the block into several micrometer thick slices should be considered. Microtomography may be necessary to precisely locate the abnormal deposit in the block. The resultant section can be deposited on different supports for physicochemical characterization, such as conventional glass slides, low-e microscope slides (MirrIR, Kevley Technologies, Tienta Sciences, Indianapolis) or kapton® film. It is only possible to collect a Raman spectrum and to observe the sample by SEM using conventional glass slides. Low-e microscope slides enable imaging of surface topology by SEM, or chemical characterization with μRaman and μFTIR spectroscopies. Note that other supports have been considered in hospitals in order to perform TEM observations at the nanometer scale.
2.3. Analysis of biological samples in a physics or chemistry laboratory
In a physics or chemistry laboratory (Figures 4 and 5), characterization using prototype experimental set ups can be performed, which may combine optics, sample holders, or detectors, developed in the laboratory. Such experiments are usually not performed in a hospital environment because the instrumentation is expensive, and the techniques are not yet on the mainstream diagnostic analysis path. The sophisticated character of these prototype setups allows significantly improved spatial resolution and/or sensitivity relative to commercial instrumentation.
Forefront physics or chemistry laboratory characterization hardware, all located in the Laboratoire de Physique des Solides, Orsay. (A) SEM apparatus sample holder. (B) New transmission electron microscope. (C) An experimental device combining XRD and XRF capabilities.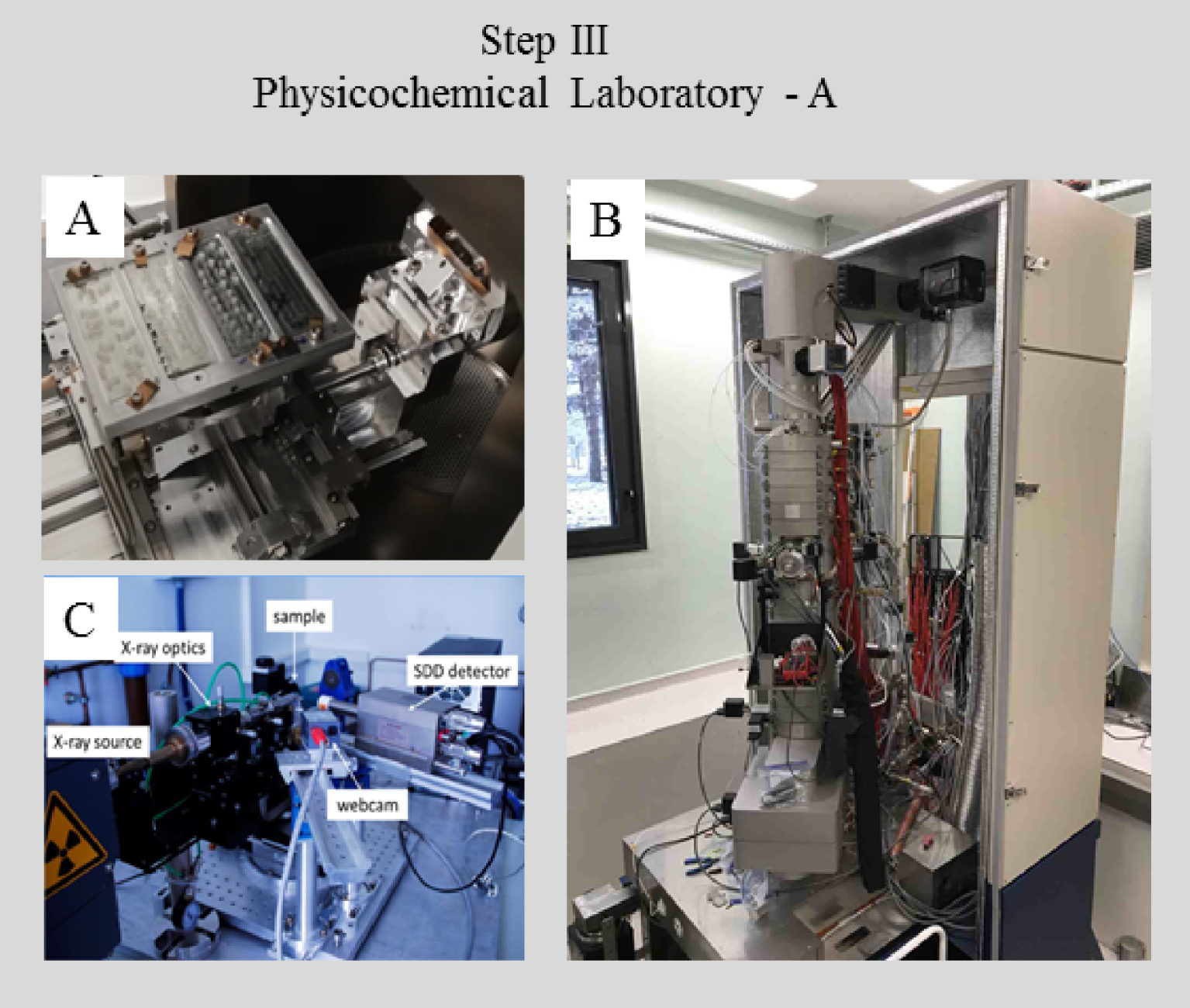
Characterization techniques available in a physics or chemistry laboratory (A) μFTIR spectrophotometer implemented at soleil (SMIS beamline); (B) and (C) AFMIR experimental set up implemented on the MUSIICS (MUlti Scale Infrared Imaging platform for Complex Systems, located at the institute of physics and chemistry) platform, (D) OPTIR (Optical PhotoThermal IR) experimental set up (SMIS beamline).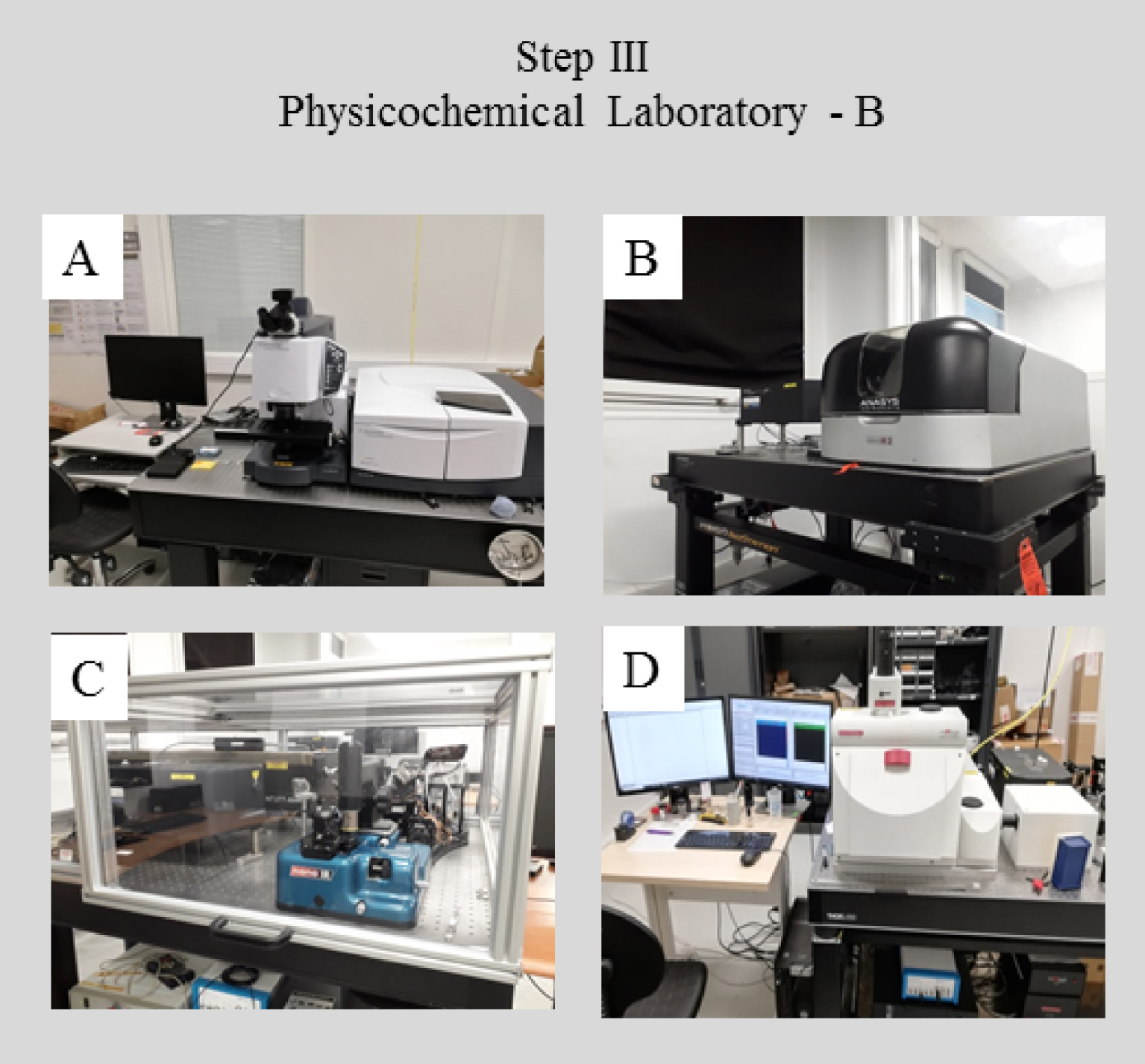
Special detectors on a TEM microscope (Figure 4B) allow nanometer scale elemental analysis by Nano-EDX as well as chemical analysis by EELS. Also, the experimental set up developed at the Laboratoire de Physiques des Solides combines a multilayer mirror and a high intensity rotating-anode generator delivering a well-defined beam (100 μm size) of sufficient intensity. Using a hybrid-pixel detector and a SDD detector, one can measure XRD and XRF signals respectively at the micrometer scale rapidly (time scale between 1 and 10 min depending on the sample) (Figure 4C).
Imaging of the topology of biopsies, deposited on low-e microscope slides and based on SEM observations and chemical characterization with μRaman and/or μFTIR spectroscopies, can be complemented by NanoIR [112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122]. NanoIR, for which two experimental configurations are possible, presents novel opportunities. The first configuration is associated with 500 nm spatial resolution and is based on a pump-probe architecture using two laser sources, one for mid-infrared excitation (the pump) and the other one for measuring the photothermal effect (the probe) [112]. The second configuration combines an atomic force microscope (AFM) and IR lasers [115, 116] and produces a spatial resolution of 10 nm [122].
2.4. Analysis of biological samples in a large scale facility
The fourth and final step in a comprehensive analysis procedure uses characterization technology usually (but not exclusively) specific to large scale instruments, which significantly increases sensitivity and/or in plane spatial resolution.
Synchrotron radiation as a submicrometer probe can be applied in several characterization techniques such as XRF or XRD. Synchrotron radiation is related to the emission of light when charged particles (electrons or positrons) moving with relativistic velocity undergo radial acceleration [123, 124, 125, 126]. Among the important advantages of a synchrotron over a laboratory X-ray source are its large spectral range, and its brilliance (a physical characteristic encompassing the photon flux, bandwidth and the angular and lateral spread of the beam) which is more than a million times higher than that of the X-ray tube. Basically, the different beamlines on a synchrotron use a selected (but tunable) part of its energy spectrum, from terahertz to hard X-ray frequencies. This confers the advantage of being able to choose the energy of the incident beam during measurements. Two facilities are implemented in France namely SOLEIL (French national source) [127] and ESRF (European Source) [128].
van der Ent et al. [129] have compared different XRF approaches using either X-ray tubes, electron, proton, and synchrotron radiation as probes. While the spatial resolution of an experimental set up using a laboratory source is around 30–100 μm, it is possible to perform similar experiments on synchrotrons with a spatial resolution well below 1 μm, and down to about 50 or even 10 nm [130, 131]. Also, there is a significant difference between the limits of detection which is >50 ppm in the case of a laboratory source and 0.1 ppm on synchrotron facilities. These detection limits depend intimately on the sample/matrix studied as well; the detection of heavy elements in tissues is much more favourable than that of light elements in a matrix of heavy elements. Experimental optimization can improve the elemental detection limits to concentrations in the range 10−9 to 10−12 g/g [132, 133].
A similar significant improvement pertains to X-ray scattering experiments. Implemented on synchrotron radiation facilities, these offer the opportunity of measurements on isolated and micrometer sized single crystals. Guo et al. [134] have shown that acquisition and assembly of complete datasets from microcrystals may be routinely carried out on synchrotron microdiffraction beamlines.
It is worth underlining that a complete new set of spectroscopies such as XAS [135, 136] or UV visible spectroscopy [137, 138, 139, 140] is available at a synchrotron radiation centre. XAS is form of spectroscopy able to describe the electronic state and the local environment of trace elements in different kinds of material including those without long range order such as nanoparticles [141, 142, 143, 144, 145, 146] and amorphous compounds [147, 148]. In a recent review, Ma et al. [149] highlighted the emergence of nanoparticles in medicine which offer novel solutions to diagnosis and treatment of chronic kidney disease.
As an example, we show in Figure 6 the different experimental beamlines we have used to characterize abnormal deposits in the human body namely Diffabs [85, 86, 150], Cristal [19, 151] and Nanoscopium [154]. On these beamlines, XRD (Diffabs and Cristal), XAS (Diffabs) or nano-tomography (Nanoscopium) experiments can be performed. Note that other beamlines such asth the Disco beamline can be of major importance [140].
Three beamline experimental stations implemented on the Soleil synchrotron (A) Diffabs (details in Refs [82, 83, 149]), (B) Cristal (details in Refs [150, 151, 152]) and (C) Nanoscopium (details Ref. [153]).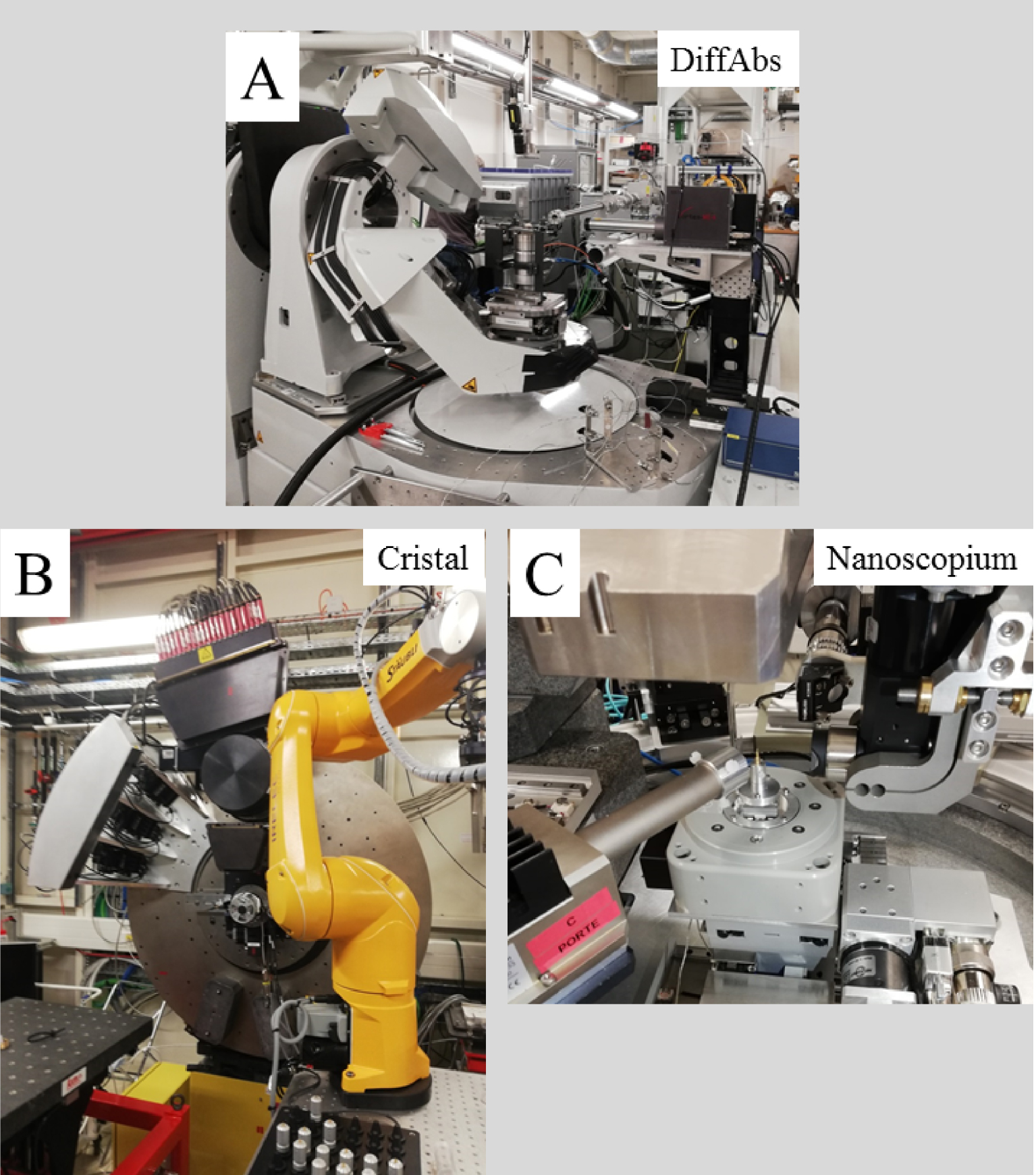
3. Sample quantity and availability
Sample quantity and availability can determine the order in which experiments are performed. Large quantities impose no priority but it must be emphasized that several scans may have to be performed several times to take possible heterogeneity into account. Obviously, if the quantity of sample is low, non-destructive characterization techniques have to be implemented first.
Sometimes, sample quantity is simply insufficient to perform the experiments. For example, XRD experiments are performed on powder; the sample is typically inserted into a glass capillary (Ø = 0.1–1 mm) and mounted on a spinner rotating at several Hz to improve particle orientational averaging. Typically, for such experiments, less than 1 μL of powder is enclosed in small glass or kapton capillaries.
Another technique needing a relatively large amount of sample is Nuclear Magnetic Resonance (NMR) spectroscopy. In order to gather information regarding local structural changes we have to take into account that the volume of a typical widely used “standard” solid state NMR sample holder (a “magic angle spinning” rotor) in a NMR experiment is around 80 μL. Also, it is possible to probe the local environment of several elements namely 1H, 13C, 31P.
4. Advantages and inconveniences of high spatial resolution
High spatial resolution experiments are not always the most desirable. Lower spatial resolution characterization can resolve many important clinical problems. We will examplify this by FTIR spectroscopy and the characterization of pathological renal calcification. In the case of kidney stones, several chemical compounds can be present in different proportions. Chemical compounds present at high abundance do not always define a medical diagnosis. Even a small proportion (5%) of struvite informs the clinician of bacterial infection. This factor imposes a requirement for an IR spectrum with a very high signal-to-noise ratio for there to be any possibility of detecting the presence of a small shoulder in the IR absorption bands or a small shift in their wavenumbers, which may help to distinguish up to 9 chemical compounds occurring in kidney stones.
It is very important to obtain a high quality IR spectrum from the core as well as the surface of kidney stones (Figure 7). Currently, only “classical” IR, in which measurements are performed on a small pellet, is capable of sufficiently high signal to noise. Also, high spatial resolution comes with characterization of only a small area of the sample. In the case of a kidney biopsy the calcification area can be somewhere within a length of typically around one centimetre and width 1 mm; it is clearly impractical to spend a few hours searching the whole sample for ectopic calcification at a μFTIR spatial resolution of 10 μm, or by NanoIR for which the area of interest is limited to 80 μm by 80 μm. Nevertheless, NanoIR experiments can represent the ultimate opportunity to determine chemical composition for very small deposits which cannot be characterized by μFTIR.
(A) Classical FTIR spectrum collected on part of a kidney stone dispersed in a KBr pellet; IR bands of various compounds present are indicated Br—brushite, CA—calcium phosphate apatite; CALC—calcite, C2—calcium oxalate dihydrate; PC—calcium palmitate; C1—calcium oxalate monohydrate, PROT—protein; TRG—triglycerides. (B) Typical dimensions of the maps which can be collected with μFTIR, OPTIR and AFMIR; the size of the probe for each is indicated. (C) Three IR spectra collected by the three experimental set ups—μFTIR (blue), OPTIR (black) and AFMIR (red).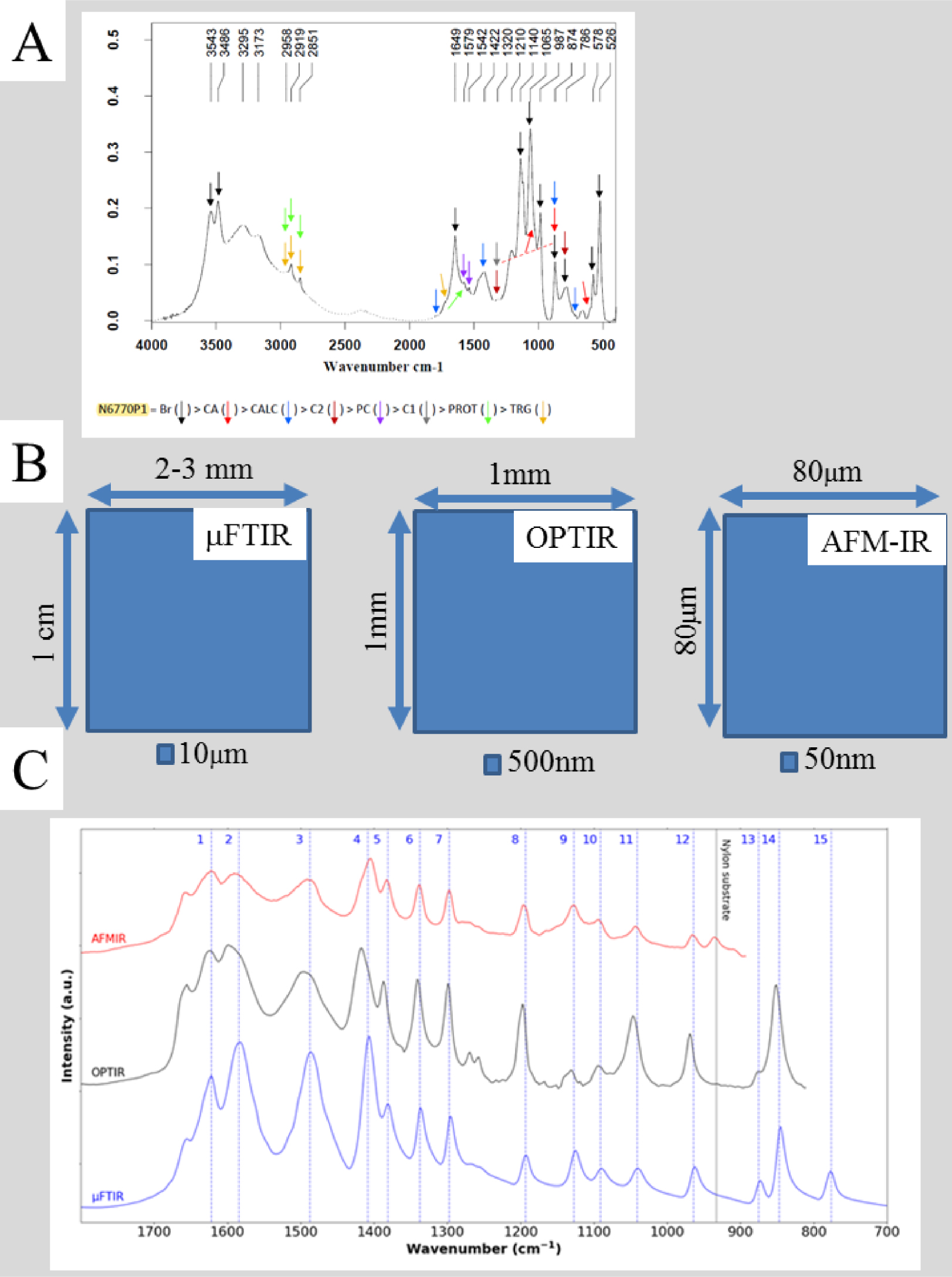
Finally, we must underline that polarization of the lasers used in NanoIR spectroscopy, either in the visible or the IR range, means that the intensity, and position of IR bands may vary, in the latter case by up to a few cm−1. This can impose limitations on the precision of any description of abnormal biopsy deposit chemistry [122].
5. Conclusion
This paper proposes a hierarchical approach to characterize abnormal deposits in biological tissues [155], starting from in vivo measurements and progressing to a physicochemical description at the nanometer scale. The approach is based on a selected set of destructive and non-destructive characterization techniques which take into account the complexity of the physicochemistry of abnormal deposits in biological tissues, consistent with their exogenous and endogenous origins. Because some of these techniques need reference compounds such an approach supposes strong interactions with research teams able to generate nanomaterials [156, 157, 158, 159]. Finally, it must be remembered that density functional theory [160, 161] offers the opportunity to relate nanomaterial atomic structure to IR, Raman or XRD characteristics [162, 163, 164, 165, 166]. As emphasized by Khosroshahi et al. [167], it’s time to use nanotechnology to change the conventional paradigm for analysis and diagnosis in cases of pathological deposits.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0