1. Introduction and scope
The term “per- and polyfluoroalkyl substances (PFASs)” refers to a wide range of organofluorine compounds that have diverse physical, chemical, environmental, and biological properties. This term was introduced in 2011 to provide a standardized language for the scientific, industrial, and regulatory communities [1]. PFASs have gained attention since the identification in the environment of two specific substances, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) [2, 3, 4]. Environmental research and regulatory efforts have since expanded to cover a considerably broader range of compounds.
PFASs are synthetic compounds that have been in production and use since the 1940s [5, 6, 7, 8]. They include small molecules and polymers and can have surface activity, hence be surfactants. The chemical and thermal stability of perfluoroalkyl moieties, along with their outstanding hydrophobic and lipophobic character, lead to countless uses of PFASs in industrial processes and consumer goods [5, 6, 8, 9, 10, 11, 12, 13, 14]. The surfactant applications take advantage of the unparalleled aqueous surface tension-lowering properties of PFAS surfactants and include use as processing aids for fluoropolymer manufacture, coatings, and aqueous film-forming foams used to extinguish fires involving flammable liquids.
It is now recognized that some PFASs are globally distributed, environmentally persistent, bioaccumulative, and potentially harmful [1, 2, 3, 15, 16, 17, 18]. PFASs can travel long distances through air, sea, and river routes, and can strongly adsorb to rocks and sediments. A 2022 study presents an alarming picture of PFAS pollution in the US, with approximately 50,000 factories either producing or using PFAS, over 4000 contaminated water treatment plants, 3500 polluted military sites, and more than 500 airports affected [19]. The French newspaper Le Monde has published a map showcasing the widespread PFAS contamination across Europe [20], serving as a stark reminder that PFAS pollution is a pressing global concern that demands action.
In 2023, the European Chemicals Agency (ECHA) published a proposal to ban PFASs [21]. This is unprecedented considering the number of substances covered by the USA Environmental Protection Agency (EPA), which has listed over 14,000 PFAS structures, as well as a list of 1915 PFAS chemicals for which no explicit structure is documented (polymers and unknown or variable composition, complex reaction products or biological materials, collectively called UVCBs under REACH) [22]. In 2023, the Organization for Economic Cooperation and Development (OECD) defined PFASs as “fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS” [23].
The present article begins by discussing the origins of the outstanding properties of PFASs, starting with the exceptional stability of the C–F bond. The second section clarifies why the widespread use of PFASs in so many industrial branches and consumer goods directly results from these characteristics, or a combination of them. The third section presents the “essential use” concept, a useful tool that could help to determine when PFASs can be phased-out. The fourth section is a brief reminder of the recent regulation efforts. A last section focuses on the outstanding tendency for amphiphilic PFASs to form supramolecular aggregates in solutions and at interfaces, and the implications of these strong self-assembling properties for both their environmental fate and remediation techniques.
2. Fluorine: nec pluribus impar among elements
Fluorine is the 13th most abundant element in the Earth’s crust (0.054% by weight), ahead of carbon (0.02%) [24]. It is primarily extracted from fluorite (CaF2, fluorspar) on a large scale, and is also present in other mineral groups, namely bastnaesite (Ca,La,Nd)(CO3)F), cryolite (Na3AlF6), sellaite (MgF2), villiaumite (NaF), fluoroapatite (Ca5(PO4)3F), topaz (Al2SiO4(F,OH)2) and various phyllosilicate, such as biotite (K(Mg,Fe)3(AlSi3O10)(OH,F)2 [25]. Use of fluorite was first described by Georgius Agricola in 1529 as an additive to metal ores to lower their melting points during smelting. The Latin verb fluere, meaning ‘to flow’, gave the mineral its name. Fluorine is scarcer in the oceans (1.3 ppm of fluoride compared to 19,000 ppm for bromine) [25, 26]. Concentrations can be higher in waters that are in contact with rocks that contain high fluorine concentrations [25]. By contrast, organically bound fluorine is very rare in Nature, with only half a dozen naturally occurring organofluorine compounds identified in plants and microorganisms; none in mammals and insects [26, 27, 28].
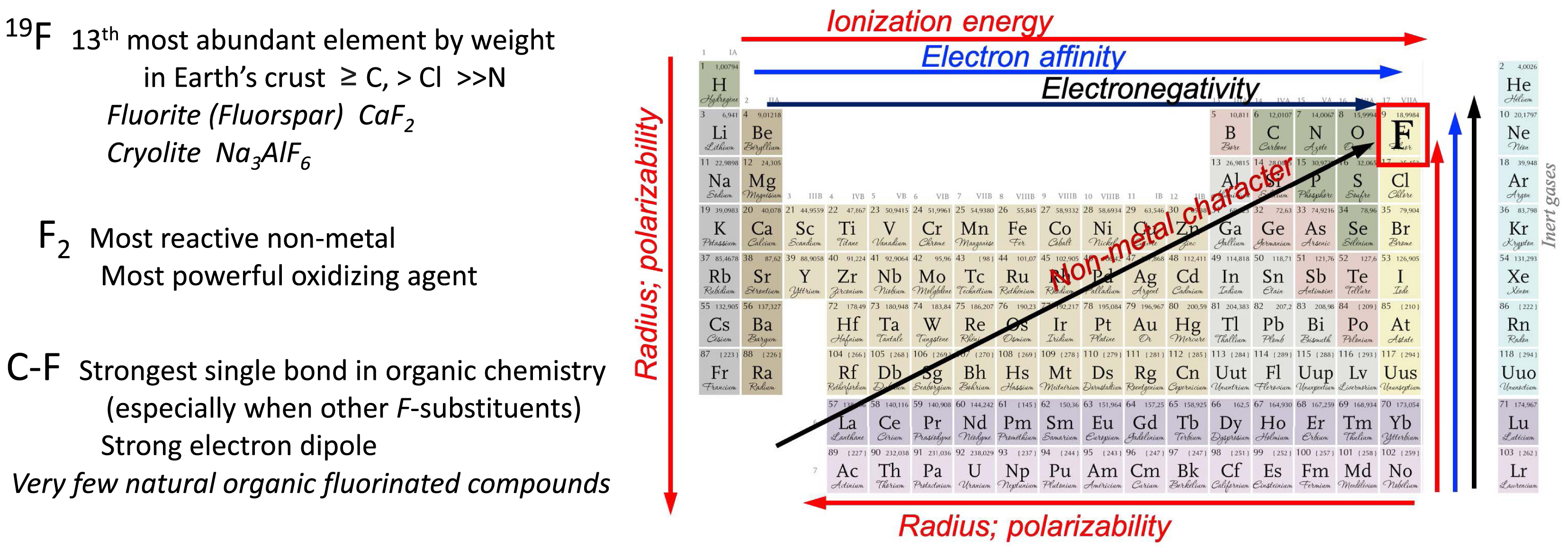
Fluorine is in pole position in the periodic table of the elements. All properties of PFASs and of the C–F bond directly derive from this extreme location.
Henri Moissan, Nobel laureate in chemistry in 1906 [29], isolated elemental fluorine F2 in 1886 using low-temperature electrolysis of potassium hydrogen difluoride dissolved in anhydrous HF. F2 is extremely reactive and a strong oxidant (highest known standard reduction potential: +2.87 V). Because of the difficulties in handling such highly reactive and corrosive reagents, organofluorine chemistry remained relatively undeveloped until the 1920s. The first perfluoroalkanoic acid, trifluoroacetic acid, was synthesized in 1922 [30], and the first perfluorocarbon, CF4, was isolated in 1926 [31]. Industrial production of F2 began during World War II as part of the Manhattan Project to produce liquid fluorocarbons and fluoropolymers for use as coolants and materials exposed to the highly reactive UF6 [32]. Commercial exploitation started with little regulation in the early 1950s, following Plunkett’s serendipitous discovery of poly(tetrafluoroethylene) (PTFE, e.g., Teflon) in 1938 [33]. After World War II, various methods were developed to control direct fluorination by F2. Vapor-phase perfluorination was initially performed by carefully and progressively adding F2 diluted in an inert gas to the hydrocarbon substrate (jet fluorination) [34]. The process was improved by starting the reaction at low temperature (LaMar process) [35, 36]. Efficient perfluorination was also obtained by adsorbing the hydrocarbon substrate onto NaF particles (aerosol fluorination) [37]. Liquid-phase fluorination provided improvements by adding the hydrocarbon substrate into F2 solubilized in an inert liquid, a fluorocarbon or a chlorofluorocarbon, followed by UV irradiation [38] or without UV irradiation (Exfluor-Lagow method) [36]. The technique was further refined by transiently grafting a long-chain fluorinated ester ligand on the hydrocarbon substrate to enhance its solubility in a fluorinated solvent (PERFECT method) [39].
Two primary methods are used for industrial-scale production of PFASs [1, 5, 7, 9, 40, 41]. The first involves the electrochemical fluorination of linear alkane sulfonic fluorides in anhydrous hydrogen fluoride (HF), known as the Simons process [42, 43]. This electrochemical process is harsh and nonselective, resulting in complex mixtures of linear, branched, and cyclic perfluorinated molecules with various chain lengths. Notably, it produces a product signature that can help trace the source of a contamination. Products labeled as PFOS typically contain 70–80% of the linear molecule. The second major production method utilizes free radical telomerization of tetrafluoroethylene (CF2=CF2) with a fluorinated alkyl iodide, most commonly CF3CF2I. This process yields a series of homologous, even carbon-numbered linear alkyl iodide telomers, CF3CF2(CF2CF2)nI [44]. Subsequent distillation allows easy isolation of pure homologs within the series, thanks to the significant molecular weight difference—about 100—between consecutive telomers (n and n + 1).
Due to its extreme top right position in the periodic table (noble gases excepted), fluorine beholds unique characteristics (Figure 1). It is the smallest of the halogens. Compared to hydrogen (1 proton, 1 electron), fluorine (9 protons, 9 electrons) has a larger van der Waals radius (1.47 Å vs. 1.20 Å) and a significantly larger single bond covalent radius (0.64 Å vs. 0.32 Å) [45].
Fluorine is the most electronegative element (3.98 on Pauling’s scale vs. 2.1 for H). It bears three lone pairs of electrons that are tightly held by the nucleus. Fluorine has thus a much denser and less polarizable electron cloud than hydrogen (polarizability 0.557 × 10−24 cm3 vs. 0.667 × 10−24 cm3).
The C–F bond is the strongest single bond known in organic chemistry (∼485 kJ⋅mol−1 compared to ∼413 kJ⋅mol−1 for C–H in hydrocarbon compounds) [46]. Due to fluorine’s high electronegativity, the C–F bond is highly polarized, with the fluorine atom bearing the partial negative charge and the carbon atom the positive charge [47, 48]. This endows the C–F bond with an electrostatic character and generates a strong electric dipole with a C–F orientation opposite to that found in C–H, which is a dramatic change. An important consequence of the highly polarized nature of the C–F bond is the availability of a low-energy σ∗ antibonding orbital that can accept electron density from nearby electron-donating groups such as lone pairs or σ bonds [47, 48]. Fluorine’s electron affinity is 328 kJ⋅mol−1 (3.40 eV) vs. 73 kJ⋅mol−1 for hydrogen. Fluorine also has a much higher ionization energy than hydrogen (1677 kJ⋅mol−1 vs. 1312 kJ⋅mol−1). The stability of the C–F bond increases with fluorine substitution (e.g., 530 kJ⋅mol−1 in CF3CF3 vs. 450 kJ⋅mol−1 in CH3CH2F). The backbone C–C bonds are significantly stronger in fluorocarbons than in hydrocarbons. One can thus consider the C–F bond as a highly polarized bond, short, strong, and unreactive. Organofluorine compounds are subject to various stereoelectronic effects, including dipole–dipole and charge–dipole interactions, and hyperconjugation.
The unique characteristics of the fluorine atom and C–F bond endow fluorinated chains CnF2n+1 (F-chains) with special properties [9, 49, 50]. Because of the low polarizability of fluorine, F-chains display lower intermolecular (van der Waals) attraction forces than hydrocarbon chains (H-chains). F-chains are also bulkier than H-chains with larger cross sections (27–30 Å2 vs. 18–21 Å2). The volumes occupied by CF2 and CF3 groups in linear fluorocarbons are 38 Å3 and 92 Å3 vs. 27 Å3 and 54 Å3 for CH2 and CH3 groups, respectively. The larger cross section of F-chains as compared to H-chains explains their larger hydrophobicity. Molecular simulations show that the extra work of cavity formation to accommodate an F-chain in water, compared to an H-chain, is not offset by enhanced energetic interactions with water [51]. The enhanced hydrophobicity of fluorinated surfaces arises because F-chains pack less densely on surfaces, leading to poorer van der Waals interactions with water. F-chains longer than 6 carbons adopt a helical conformation rather than the all-trans planar zig–zag conformation characteristic of alkyl chains [50, 52, 53]. This particularity was recently re-assigned to hyperconjugation stabilization through
3. PFAS’s exceptional properties produce a wide scope of applications
Very different compounds are grouped under the PFAS denomination that have distinctive properties and, consequently, different uses. Recent reviews have provided eloquent images of the “complex PFAS universe” featuring the intermediates and final manufacturing products [10, 12, 13]. A first distinction must be made between polymers and substances of low molecular weight. In each class, it is then important to differentiate the compounds that display substantial surface activity from those that do not. For all of them, fluorine contributes to creating or enhancing unique properties and has, for this reason, been called “the enabler” [55].
3.1. Fluorocarbons
Fluorocarbons (CnF2n+2) are PFASs that are devoid of polar head. They embody the essential properties of F-chains [41, 50, 56, 57, 58, 59]. The biological inertness of liquid fluorocarbons and closely related, essentially non-amphiphilic derivatives (e.g., perfluorooctylbromide, perfluoroethers, or amines) is well documented. These compounds, formulated as nanoemulsions, were investigated as injectable oxygen carriers (temporary blood substitutes) in Phase III clinical trials in Europe and the USA [41, 56, 60, 61, 62]. Fluorocarbons can solubilize large amounts of gases (e.g., up to ∼50% v/v of O2, while O2 solubility in water is only ∼2.5% v/v), which is a direct consequence of their low van der Waals interactions. More generally, fluorocarbon nanoemulsions have the potential to alleviate hypoxia in therapies that benefit from oxygenation, such as photodynamic therapy, radiation therapy, sonodynamic therapy and immunotherapy [63, 64, 65, 66]. They are also actively investigated as 19F MRI contrast agents (due to high 19F nuclei concentration and absence of protons [60]), as ultrasound contrast agents (microbubbles, as well as phase-change nanodroplets [60, 67, 68]), as 18F positron emission tomography (PET) contrast agents and multimodal contrast agents [66, 69]. Liquid fluorocarbons have been used in biotechnology to supply oxygen and other gases to cells in cultures [70]. Due to weak van der Waals interactions, fluorocarbons have the lowest surface tensions, refractive indices, and dielectric constants of all liquids. They have higher density, fluidity, compressibility, gas solubilities, and critical temperatures than hydrocarbons of similar length. As a consequence of their outstanding hydrophobicity, their solubility in water is extremely low and decreases exponentially with chain length. Fluorocarbons are chemically inert, in particular nonflammable, and can withstand very aggressive conditions (temperature and pH, think of UF6). They have found numerous applications as functional and heat transfer fluids in the electronics industry and in energy storage.
3.2. Fluorinated surfactants
Fluorinated surfactants comprise an F-chain covalently bound to a polar head. An alkyl spacer (e.g., –(CH2CH2)–) is often inserted between the hydrophilic and hydrophobic moieties. Fluorinated surfactants are generally more effective (they reduce the surface tension of water to lower values) and more efficient (lower concentrations are needed to reach lower surface tensions) than hydrocarbon surfactants [61, 71, 72]. The incremental change in free energy of adsorption at the water/air interface per CF2 group is −5.1 kJ⋅mol−1 as compared to −2.6 kJ⋅mol−1 for a CH2 group. They are superhydrophobic and lipophobic; in other words, they repel oils as well as water. Their mechanical, optical, and electrical properties are unrivaled.
Table 1 collects some PFASs among the better-known and most used. First came PFOS and PFOA, and the higher homolog, perfluorononanoic acid, PFNA, which are often called “legacy PFASs”. Shorter-chain derivatives, such as perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA) and their precursors, fluorotelomers C4F9(CH2)2OH (4:2 FTOH) and C6F13(CH2)2OH 6:2 FTOH, were subsequently used in an attempt to reduce toxicity and environmental persistence [73]. Toxicological evaluation provided surprises. Although the blood elimination half-lives of PFASs generally decrease with chain length, PFHxS has a longer half-life in humans than PFOA or PFOS. Likewise, although longer-chain PFASs tend to have longer renal elimination half-lives in rats, PFBA has a slower renal clearance than PFHxA. These short PFASs tend, in general, to be more soluble in water and have a lower potential for sorption to particles than the long-chain ones. They have, however, a higher potential for being transported on long distances by waterways. Compounds in which the fluorinated chain is interrupted by oxygen atoms have also been introduced to facilitate their degradation. Unfortunately, some of these compounds were eventually found to be more toxic than PFOA [74]. These are examples of regrettable substitutions that must be avoided in the future. More generally, the pharmacokinetics and (eco)toxicological profiles of PFASs are characterized by still poorly understood species, sex, and other variations.
Chemical formula of some legacy PFASs and of compounds developed more recently
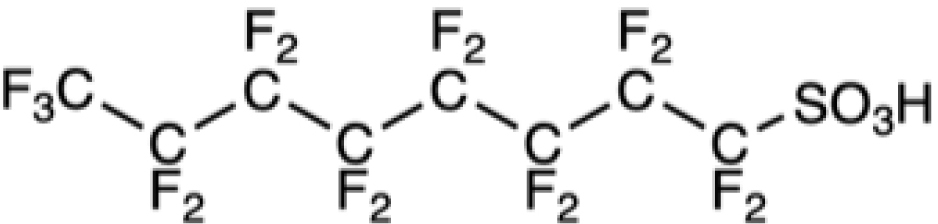
PFOS |
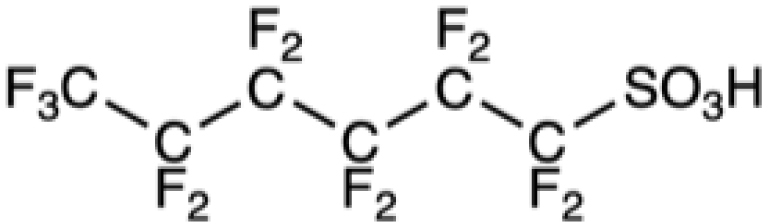
PFHxS |
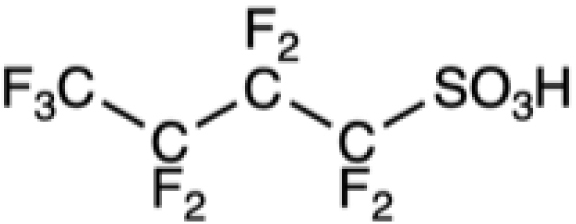
PFBS |
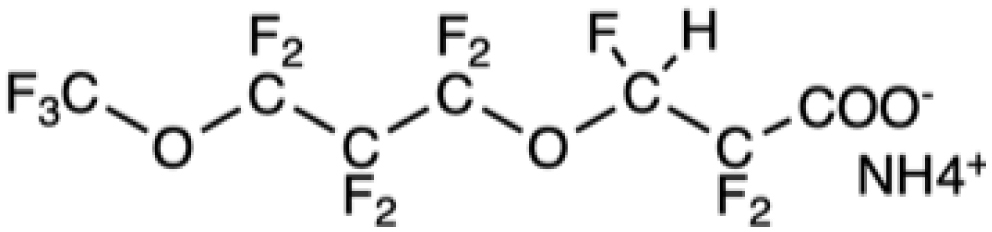
ADONA |
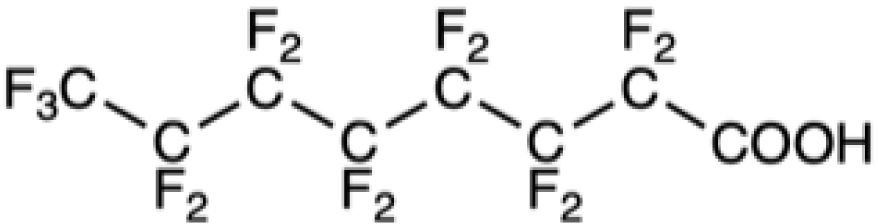
PFOA |
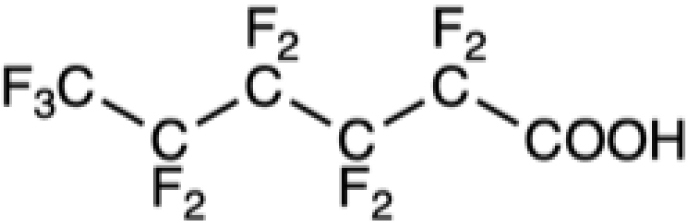
PFHxA |

PFBA |
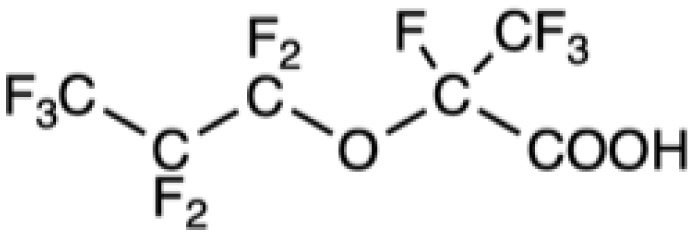
HFPO-DA |
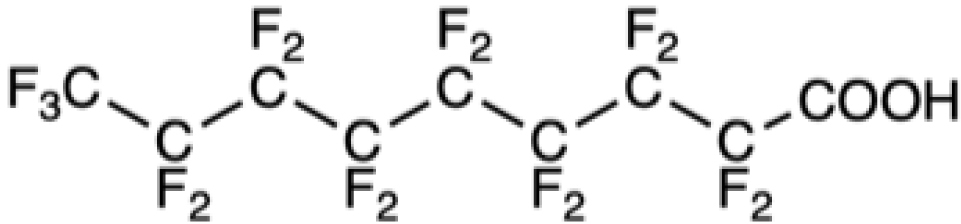
PFNA |

PFO3OA |

PFPrA |
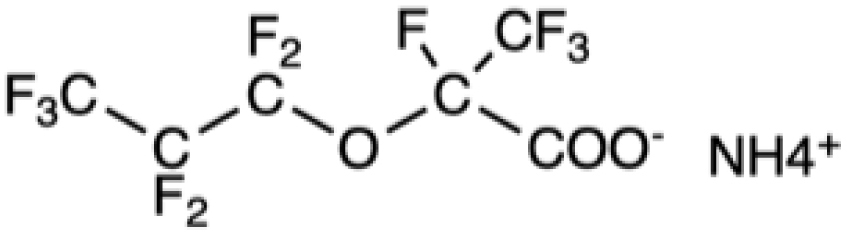
GenX |
Due to their unmatched properties, PFAS surfactants have found uses in numerous industrial processes (Figure 2). Selected examples include uses as processing aids in the production of fluoropolymers (PTFE and polyvinylidene fluoride, PVDF), in paints and varnishes, medical devices, and in the aerospace industry (aircraft brakes and hydraulic fluids). They are used to inhibit corrosion in the machinery and equipment industry, including treatment and coatings of metals (metal plating) and as mold release agents; in the high-technology sector, as in electronics and semiconductors, for example, as additives to photoresist formulations, energy, and electric vehicles. Surfactant PFASs are used for protection and improved security, for example, to protect from fire (fire-fighting foams, firefighter protective gears) and contamination (medical workwear), in lubricants, coatings (antireflective, antifogging or antistatic, …) adhesives, pesticides, etc. Finally, they are used in countless consumer and comfort products, such as for waterproofing and as stain-repellent treatment of fabrics, furniture, upholstery, cloth, carpet, leather, paper, and cardboard (food packaging).
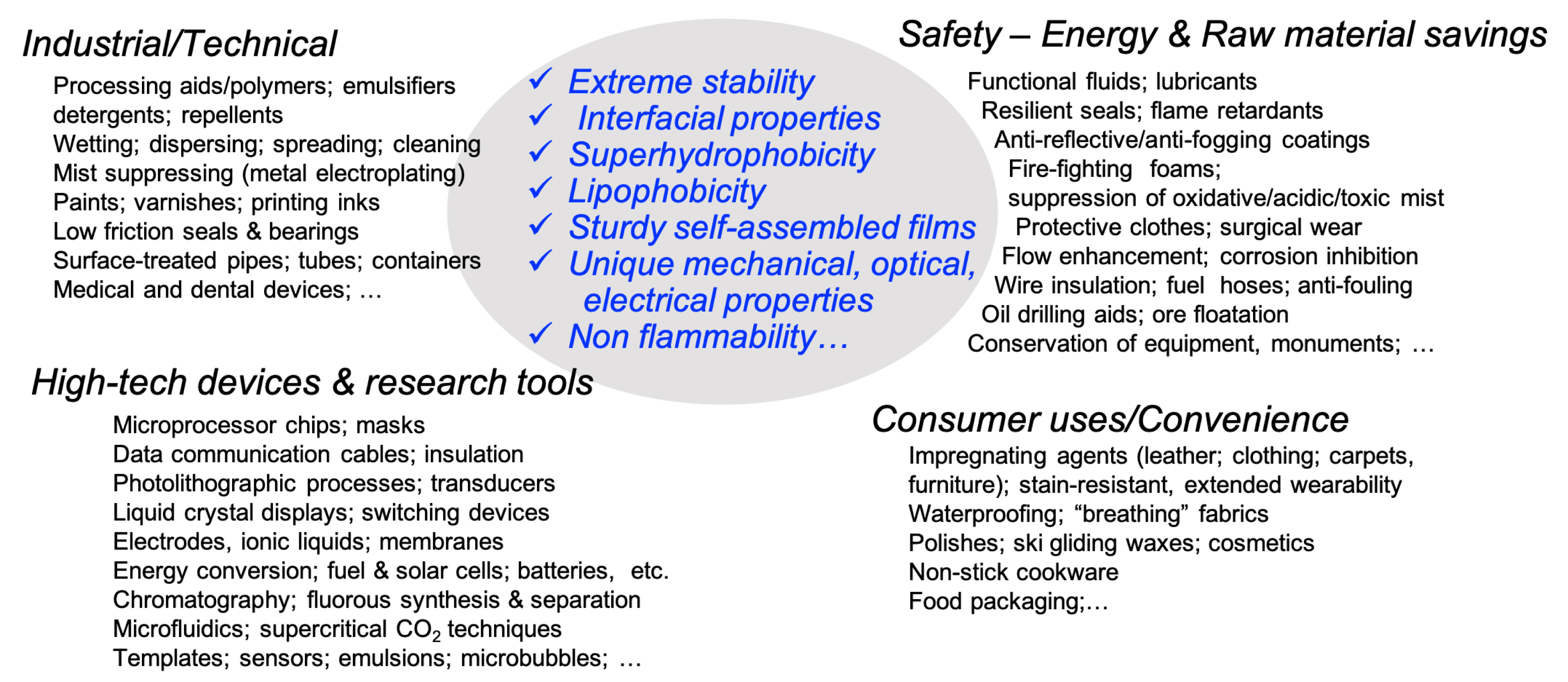
PFAS’s specific features are exploited in hundreds of uses ranging from essential to futile.
3.3. Fluoropolymers
Concerning polymers, there are two different situations to consider: those that use fluorinated monomers and those that require a PFAS, initially an ammonium salt of PFOA or PFNA, as a processing aid in emulsion polymerization to achieve a homogenous, fine particle size distribution. Traces of PFAS surfactants may then remain even after curing of the material.
Notably, technologies currently exist to synthesize key fluoropolymers such as PTFE, PVDF, and fluorinated elastomers (FKM) without using PFAS surfactants as processing aids [75]. Fluoropolymers combine the many advantages of fluorocarbons, such as hydrophobicity and lipophobicity, excellent chemical stability and bio-inertness, low surface energy, and aptitude for phase segregation, to those of polymers that are well established in materials science [76]. Fluoropolymers are resistant to acids, bases, oxidation, corrosion, and heat. They have low refractive index, flammability, and surface energy. Fluoropolymers have diverse structures ranging from semi-crystalline to amorphous and can provide thermoplastics, thermosets, and elastomers. Many fluoropolymers can be recycled [77]. Fluoropolymers are being used for high-performance materials in a wide range of industries, including the automotive, aerospace, chemical, nuclear industries, electronics, and medical industries, and participate in green economy initiatives [78]. Variously nanostructured fluoropolymers yield superhydrophobic and chemically resistant surfaces when deposited on solid surfaces that are minimally wetted by water and organic solvents, and could be used for water collection, antifouling, and self-cleaning applications. Some fluoropolymers (perfluorosulfonic acid membranes, Nafion, Aquivion) are used in the important industrial chloralkali process, the large-scale industrial process that produces common chemical feedstocks, such as chlorine gas and sodium hydroxide [79, 80], for fuel cells and energy storage (lithium-ion batteries). PVDF and fluorinated ethylene–propylene FEP, a copolymer of hexafluoropropylene and tetrafluoroethylene, are used as binders for the anode and cathode in nearly all commercial batteries [81]. Besides their well-known applications in medical coatings and implants [82], fluoropolymers have found advanced uses in biomedical applications during the past decade, including in gene delivery, cytosolic protein delivery, drug delivery, magnetic resonance imaging, photodynamic therapy, anti-fouling and anti-bacterial applications, and tissue engineering [78, 83].
4. The concept of essential uses
It soon became obvious that determining when PFASs are not essential and when alternative solutions existed was necessary. It is noteworthy that in the revised OECD definition of PFASs, “the term “PFAS” does not inform whether a compound is harmful or not, but only communicates that the compounds under this term share the same trait for having a fully fluorinated methyl or methylene carbon moiety” [23]. Discrimination between essential and non-essential uses seems to be the key to effectively managing substances of concern [84, 85]. To be considered “essential”, a use must be “necessary for health, safety or is critical for the functioning of society” and “there are no available technically and economically feasible alternatives” as defined under the Montreal Protocol (Decision IV/25.19).
Most fluorinated pharmaceuticals fall in the latter category (category 3, Table 2, Figure 3). Fluorine-containing compounds now constitute a quarter of all small-molecule drugs in the pharmaceutical market and are found among the most prescribed and top-selling drugs [86]. They are particularly efficient in the treatment of cancers and neurodegenerative diseases (Parkinson’s and Alzheimer’s diseases) Fluorination of pharmacological agents habitually enhances their pharmacological effectiveness, increases their biological half-life, and improves their biosorption [87]. Most fluorine-containing pharmaceuticals only contain one or two fluorine atoms. Some drugs contain one or two CF3 groups or a short perfluoroalkyl moiety. A semi-fluorinated alkane, perfluorohexyloctane C6F13C8H18 (Miebo™), was approved in 2023 by the Food and Drug Administration (FDA) as a single-component eye drop to reduce tear evaporation in the treatment of the dry eye disease [86]. It is noteworthy that the active ingredients in pharmaceuticals and pesticides are regulated under regulations other than REACH and benefit from derogations in the EU restriction proposal. In view of the risk associated with emission (particularly in the case of plant protection products, the derogation comes with a recommendation to the EU to address these concerns in the respective regulations to reduce the use and emissions of PFAS as much as possible.
The “essential use” concept can help phase-out certain PFASs
| Category | Definition | Examples of use |
|---|---|---|
| 1. Non-essential | Uses that are not essential for health or safety and the functioning of the society. Uses essentially driven by market opportunity | Personal care products and cosmetics, ski waxes, cooking ware, water- and stain-free textiles, leisure clothing |
| 2. Substitutable | Uses regarded as essential because they have important functions, but for which alternatives have been developed that have adequate performance | Class B fire-fighting foams, perfluorosulfonic acid membranes for fuel cells |
| 3. Essential | Uses considered as essential for health or safety and the functioning of the society and for which alternatives are not yet established | Fluorinated drugs (regulated under other regulations than REACH), perfluorosulfonic acid membranes for the chloralkali process, medical devices, occupational protective clothing |
Adapted from [84] under CC BY-NC 3.0.

Some uses of PFASs that are considered essential for safety (to protect from fire and contamination), health (drugs, safe and functional implants, medical coatings), and chemical (key processes), energy storage and aerospace industries.
Another case of essential use is exemplified by perfluorosulfonic acid (PFSA) polymer-based membranes (Nafion®, Chemours, formerly DuPont) used in the chloralkali process (Figure 3) [79, 80]. These thermoplastics are used in a wide range of chemical synthesis and separation procedures, and in analytical instrumentation [88, 89]. Nafion® provides the excellent conductivity of ions necessary for the electrochemical process while operating under highly corrosive conditions. It has replaced other processes that used more energy and generated hazardous materials and byproducts such as asbestos or mercury [90]. The use of PFSA membranes in the chloralkali process is thus considered essential (category 3, Table 2). On the other hand, the use of PFSA membranes as proton exchange membranes for high-efficiency fuel cells is considered substitutable. Lithium-ion batteries cannot operate without PFAS today [91]. Moreover, PFAS are indispensable in semiconductor manufacturing; a ban on these substances would threaten the progression of innovative technologies [91].
Since their introduction in the 1950s, side-chain fluorinated polymers have provided liquid repellency to many textile products. Their uses range from nice-to-wear property in daily clothing to essential protection in occupational protective attire (Figure 3) [92]. Medical textiles are examples in which the technical standards for the protection of human life (EU Medical Devices Directive 93/42/EEC) may be challenging to meet without PFAS. Similarly, meeting the performance standards set by the USA National Fire Prevention Association for protective clothing for firefighters seems difficult without fluorinated compounds.
Class B fire-fighting foams, designed to extinguish fires of flammable liquids, such as hydrocarbons, contain either PFASs or proprietary mixtures of hydrocarbon or silicone surfactants. Many companies have developed fluorine-free class B foams that meet the standard fire-fighting performance certifications [93]. There is some debate about whether PFASs are still needed in certain situations, such as fires occurring in refineries. Many airports, chemical industry facilities, and national defense forces have already switched to fluorine-free foams. Of note, the silicone-based foams may contain residual amounts of cyclic siloxanes that are also listed as Substances of Very High Concern (SVHC) under REACH.
At the other end of the scale, the use of PFASs in personal care products and cosmetics (waterproof makeup, hair products, skin creams, etc.) is considered as non-essential (category 1, Table 2, Figure 4). A wide range of PFAS surfactants and PTFE are concerned [94]. The use of PFAS in these products leads to direct human exposure and potential health effects following dermal or oral uptake. According to a recent OECD report, many companies have phased out PFASs, suggesting that non-fluorinated alternatives can be or are already found [14].

Some extensive, high-tonnage everyday convenience PFAS uses. Good to have, but indispensable?
5. Recent regulations and voluntary actions
Since the phase-out of PFOS by 3M in 2000, important regulatory actions have been decided, and some of them have come into effect. In particular, the European REACH legislation has considerably restricted the use of long-chain PFAS and has listed certain short-chain compounds as “Persistent, Mobile and Toxic” (Table 3).
Recent regulations undertaken to restrict PFASs
| 2006: | -PFOS ban (restriction moved to EU POP Regulation) | |
| 2019: | -TDFAs in solvent-based spray applications (Annex XVII entry 73) | |
| 2020: | -PFOA, salts and related substances (restriction moved to EU POP Regulation) | |
| 2021: | -C9–C14 PFCAs (Annex XII entry 68) and PFHxS and related substances (proposal expected to be included in EU POP Regulation) | |
| 2022: | -Aqueous firefighting foams (proposal in preparation) | |
| 2023: | -PFHxA salts and related substances (proposal waiting for decision making) | |
| SVHC identification | 2012: | -C11–C14 PFCAs listed as very persistent and very bioaccumulative (vPvB) |
| 2013: | -PFOA (+salts) listed as persistent, bioaccumulative and toxic (PBT) | |
| 2015–16: | -PFNA and PFDA (+salts) listed as PBT | |
| 2017: | -C6 PFCA (PFHx + salts) listed as vPvB) | |
| 2019: | -2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propionic acid (+ salts and acyl halides) listed as Persistent Mobile and Toxic (Equivalent Level of Concern (ELoC)) | |
| 2020: | -C4 PFSA (PFBS + salts) listed as ELoC | |
| 2022: | -Perfluoroheptanoic acid (PFHpA) + salts listed as PBT, vPvB and ELoC | |
| On-going | 2023: | -ECHA PFAS ban proposal Annex XV |
| 2024: | -USEPA Significant New Use Rule (SNUR) list of PFAS listed on TSCA (Toxic Substances Control Act) inventory |
TDFA: C6F13(CH2)2Si(OH)3, PFCA: perfluorocarboxylic acid, PFSA: perfluorosulfonic acid. Adapted from [21].
The European Chemical Agency (ECHA) is presently examining a more global restriction project. Derogations are proposed for certain PFASs that are considered essential and bring decisive benefits to society, in particular in medicine. Other countries also adopt some regulations, like the United States Environmental Protection Agency, which now has inventoried certain PFASs on a list of toxic substances.
6. The propensity of PFAS to self-assemble and its likely impact on their environmental fate and remediation methods
Amphiphilic PFAS, like any surfactants, were reported to self-assemble in aqueous solutions to form micelles above the critical micellar concentrations (CMCs). CMC decreases, and micelle size increases with increasing surfactant’s F-chain length and in the presence of an electrolyte [99, 100, 101]. The presence of organic compounds, such as ethanol, decreases the CMC. However, fluorinated surfactants have an exceptional aptitude for forming not only micelles but a wide variety of more ordered supramolecular assemblies such as liposomes, fibers and fibrils, and ribbons that can be extremely robust (Figure 5).
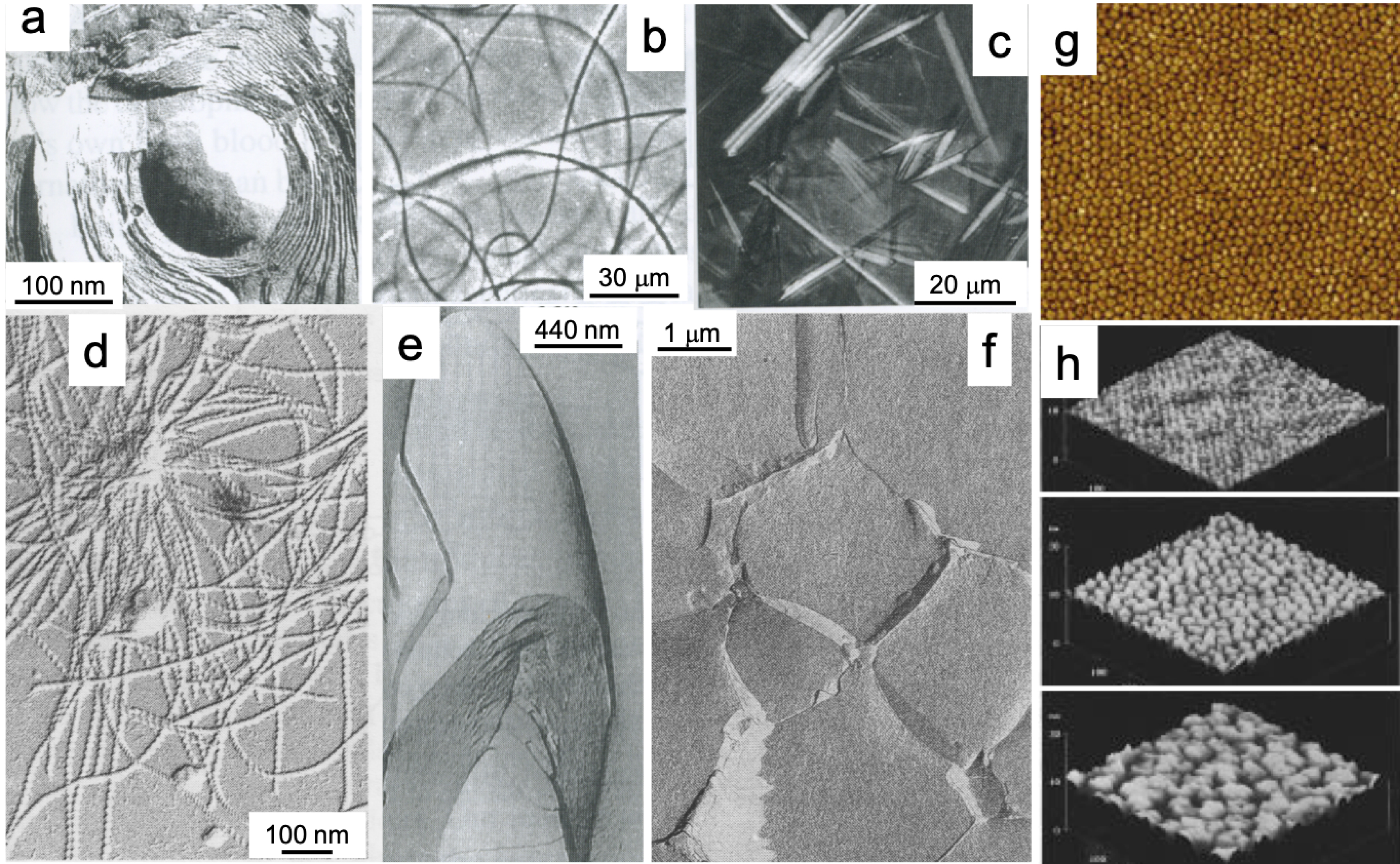
PFASs self-assemble in aqueous solutions to form large and robust supramolecular assemblies that hydrocarbon surfactants do not form. (a) Liposomes formed by a single-chain phosphatidylcholine surfactant (adapted from [71]); (b–e) flexible and rigid fibers formed by dimorpholinophosphates and glucophospholipids [71, 95], (f) polyaphron gels formed with an amine oxide fluorosurfactant [96]. Structured nanodomains formed onto solid surfaces by (g) semi-fluorinated alkanes (image size: 1 × 1 μm2) [53, 97] and (h) semi-fluorinated carboxylic acids (image size: 500 × 500 nm2 height: 20 nm) [98]. Masquer
PFASs self-assemble in aqueous solutions to form large and robust supramolecular assemblies that hydrocarbon surfactants do not form. (a) Liposomes formed by a single-chain phosphatidylcholine surfactant (adapted from [71]); (b–e) flexible and rigid fibers formed by dimorpholinophosphates and glucophospholipids [71, ... Lire la suite
This specific behavior is supported by a large corpus of data collected on fluorinated surfactants having a wide variety of structures with different F-chain lengths and polar heads, such as sugars and polyols, aminoacids, amine oxide and morpholinophosphates [61, 71, 102]. As a striking example, a single-chain fluorinated phosphocholine derivative can form discrete bilayered structures (liposomes, vesicles) that are extremely stable (e.g., they can resist heat sterilization) [103, 104], while its hydrocarbon analogs only form micelles. Even more organized, quasi-crystalline tubular aggregates were obtained from a single-chain derivative of dimorpholinophosphate [105, 106] and from anionic glucophospholipids [107].
Semi-fluorinated alkanes were found to form nanometric domains at the surface of solid surfaces spontaneously [53, 97, 108, 109]. Similar nanodomains were obtained with fluorinated carboxylic acids [98]. These systems are extremely robust and often quasi-crystalline, a behavior that contrasts sharply with that of hydrocarbon surfactants which generally only afford labile micellar solutions. Self-assembled nanodomains were also found to form at the surface of water [53, 110, 111, 112], affording highly viscoelastic 2D gels [113].
The capacity of PFAS surfactants, especially long-chain ones, to adsorb on solid surfaces and at fluid interfaces has become a hot topic because PFAS pollutants adsorb on rocks and sediments. Natural and artificial soils (e.g., concrete, tarmac, asphalt, and others) are heterogeneous environments that can contain pockets of air and water and, when contaminated, organic matter (nonaqueous phase liquids, NAPL) [114]. These environments are very complex and still poorly understood [115].
Given the known physical chemistry of PFAS surfactants, it is foreseeable that, even if their concentrations in the environment are low, PFASs will concentrate at any gas/solid, gas/liquid, and liquid/liquid interfaces present in the environment. At these interfaces, they will likely form such supramolecular aggregates that may act as PFAS reservoirs, which in turn may affect their fate in their environment and also interfere with certain remediation processes. Our understanding of these effects and possible interferences is still essentially inexistent. Considering the self-aggregation behavior of PFASs is therefore indispensable. One prime example concerns the PFAS contamination by firefighting foams. Many sites, particularly airports, are heavily contaminated because they have served as training sites for many years by firefighters who use PFAS-based extinguishing foams. Understanding what types of supramolecular aggregates form under these conditions, their behavior, and their stability is mandatory to help develop effective remediation methods.
7. Concluding remarks
As a result of incautious, extensive usage, PFASs are now found all over the planet. Longer-chain perfluoroalkylated surfactants are very persistent, mobile, bioaccumulative, and biomagnifying. Exposure of the biosphere, including human and animal populations, via food, beverages, wastewater, air, dust and soil has become ubiquitous.
Some of these chemicals, such as PFOA and PFOS, have been associated with untoward biological effects, including liver toxicity and reduced response of children to vaccines. Other potential risks concern renal and kidney cancers, reduced birth weight, increased cholesterol level and the propensity of respiratory tract infections, thus raising legitimate concern and calling for close regulation.
PFASs should obviously be phased out when not indispensable or when safer and similarly effective functional options exist. Treating PFASs as a single class and applying a global ban on them (and consequently on their outstanding specific performances) seems unsatisfactory and inappropriate, given the vast diversity of structures, properties, behaviors, potential for bioaccumulation, and toxicity observed. Such a drastic approach would also change the traditional way chemicals are regulated. The proposed European ban on over 14,000 PFASs raises many concerns and carries unintended potential negative consequences. Given their much lower bioavailability and toxicity, there is no strong scientific basis for the inclusion of fluoropolymers in the ban. Active fluorinated pharmaceuticals (not excipients) for which the risk/benefit ratio is significant and the tonnages are relatively small are regulated separately. Regulation needs to consider both the technical difficulties encountered in replacing PFASs and society’s expectations regarding product performance.
The search for less toxic, more environmentally friendly alternatives to PFASs that display similarly high effectiveness for a given function is imperative but challenging, if only because their outstanding properties derive directly from fluorine’s location in the periodic table. It is also indispensable that the biological and environmental toxicities of any alternative chemical be thoroughly investigated prior to implementation so as to avoid regrettable substitutions.
Fortunately, many applications can do with lesser performances and PFAS-free substitutes have been devised and are being successfully implemented. For example, PFAS-free fire-extinguishing foams have been developed to replace those that have contaminated many airports, particularly in the USA.
Substantial remediation efforts are definitely in order. Decontamination of polluted sites requires the elaboration of dedicated detection, monitoring, and identification techniques, and particularly of inexpensive and portable instrumentation. The development of effective remediation techniques also implies better consideration and understanding of the propensity for PFASs to self-assemble in sturdy aggregates, and of its dynamics. Likewise, for their probable impact on PFAS destruction.
Greater scrutiny is mandatory if we wish to preserve the unique advantages and benefits of using PFASs when no alternatives yet exist. Many high-efficacy niche products have negligible environmental impact. The “essential use” concept should help sort out specific high-performance-demanding uses for which no non-fluorinated alternatives have yet been found, from uses for which sufficiently effective substitutes have become available that fulfill the demanded function, and from convenience uses that are definitely not essential, and should thus help phase out non-essential uses. Like toxicity, environmental dangerousness is a matter of dosage/amount released. Altogether, the evaluation of PFAS essentiality is a formidable task in view of the number of chemicals to be scrutinized and of the often-insufficient public information available on the structure, performances, tonnages, environmental behavior, and toxicity of certain industrial products. It also supposes increased exchanges and cooperation between industry, academia, regulatory agencies, research institutions, and civil society.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Note added in proofs
PFAS no longer “eternal” pollutants? In a remarkable, newly published paper, Gouverneur et al. [116] describe a ground-breaking mechanochemical approach for the complete destruction of PFAS that involves the mineralization of the PFAS, once collected, through reactions with potassium phosphate salts. Additionally, this technology enables the recovery and reuse of fluorine to synthesize new high-value fluorochemicals. PFAS could thus become partners in a virtuous circular and sustainable fluorine chemistry.






 CC-BY 4.0
CC-BY 4.0