1 Introduction
Since a couple of years, the late metal transition catalysis has raised an increasing interest. Indeed, the research activity in this field has exploded with the discovery of highly active diimine palladium- and nickel-based complexes 〚1–3〛, which yield moderately to highly branched polyethylenes 〚4,5〛. More recently, Brookhart and Gibson have described the synthesis of new efficient pyridine bis(imine) iron- or cobalt-based catalysts for the oligomerisation or the polymerisation of ethylene and propylene 〚6–17〛. Some of them have revealed extremely active for the synthesis of linear polyethylenes. These complexes have also been studied by some other groups 〚18–23〛 and it was shown that isotactic polypropylene (65% mm dyads) could be obtained 〚18〛. In this paper, we report the synthesis of new catalysts, based on the Brookhart and Gibson complexes. The influence of the ligand substituents on the catalytic activity has been investigated, and the ability of these complexes to copolymerise ethylene and 1-hexene will be discussed.
2 Results and discussion
Several iron and cobalt complexes have been synthesized (Fig. 1). Complexes 1–4 have already been described 〚7,9–11〛, but complexes 5–8 have never been synthesized before. For the latter, the aryl groups have been substituted by halogen atoms, either in the ortho- or in the para- position. The halogen atoms, which are electron-withdrawing groups, should diminish the electron density on the metal. Thus, the olefin coordination should be accelerated and as a consequence a higher catalytic activity should be observed.

Iron and cobalt complexes.
The main results for the polymerisation of ethylene are summarized in Table 1. Methylaluminoxane was used as the activator for all the experiments. As it can be seen, iron-based catalysts are generally more active than their cobalt analogues, except in the case of the brominated ligand (complex 5 versus 6). Indeed, in this case, the cobalt complex is as active as its iron analogue.
Ethylene polymerisation with iron and cobalt catalysts.
| Catalyst | Loading (μmol) | Yield (g) | Activity (g mmol–1 bar–1 h–1) | |
| Fe | 1 | 10 | 4.7 | 930 |
| 3 | 9 | 4.6 | 1010 | |
| 5 | 10 | 4.5 | 980 | |
| 7 | 9 | 4.9 | 1110 | |
| Co | 2 | 10 | 3.3 | 650 |
| 4 | 9 | 0.5 | 100 | |
| 6 | 10 | 4.3 | 940 | |
| 8 | 9 | 1.3 | 300 |
Besides, for the iron complexes, the presence of an heteroatom on the ligand has very little effect on the catalytic activity, which remains always in the same range (900–1100 gPol mmolFe–1 h–1 bar–1). On the contrary, for the cobalt complexes, the substituents on the aryl group have a huge effect on the catalytic activity, which increases from 100 (complex 4) to around 1000 gPol mmolCo–1 h–1 bar–1 (complex 6). In particular, the presence of a bromine atom has a very positive effect. The electronic density of this metal seems to be more affected by a modification of the aryl groups substitution.
All the samples are insoluble in classical organic solvents, confirming the linearity of the polyethylenes. The molar mass of some of these polymers has been measured by high temperature Size Exclusion Chromatography (SEC). As already described by Gibson et al. 〚10〛, for such Al/Met ratios, the molar mass distribution is always bimodal (MWD > 15) and the molar masses are low (Mn ≈ 7000 g mol–1).
The thermal properties of these polyethylenes have been investigated by Differential Scanning Calorimetry (DSC). The results are displayed in Table 2. For each polymer, two or three melting temperatures have been detected, probably corresponding to the different polymer families observed by SEC. The cristallinity is almost the same for all the polymers obtained with the iron complexes and close to 0.8, which is in agreement with a linear structure. For the polyethylenes obtained with the cobalt complexes, the cristallinity is a bit lower. What should also be noted is the difference observed for the highest melting temperature for the different polymers. For the PE obtained with the iron complexes, it is around 126 °C, whereas with the cobalt complexes, it is below 110 °C (except in one case). This difference is hard to explain, but it is probably related to the difference observed for the cristallinity. Nevertheless, the highest melting temperatures we have measured are quite low compared to ‘standard’ linear polyethylenes (Tm > 130 °C), probably because our polyethylenes contain low molar masses polymers.
DSC analysis of the polyethylenes.
| Catalyst | Tm (°C) | ΔH0 (cal g–1) | χc | |
| Fe | 1 | 98, 126 | 54.4 | 0.78 |
| 3 | 96, 127 | 56.2 | 0.80 | |
| 5 | 99, 125 | 58.2 | 0.83 | |
| 7 | 78, 100, 126 | 44.6 | 0.64 | |
| Co | 2 | 94, 105, 112 | 51.4 | 0.73 |
| 4 | 140 | 57.3 | 0.82 | |
| 6 | 95, 105, 112 | 47.2 | 0.67 | |
| 8 | 70, 101 | 53.3 | 0.76 |
The influence of the polymerisation temperature on the catalytic activity has also been investigated. Three examples are displayed in Fig. 2. With all complexes, a bell curve was obtained. The catalytic activity increases for the lowest polymerisation temperatures, reaches a maximum around 30 °C and decreases for the highest temperatures. Moreover, it can be noticed again that in the case of the brominated ligand, the catalytic activity of the cobalt and the iron complexes are very close and the catalytic activity of the cobalt catalyst is even higher than that of the iron one for the lowest temperatures.
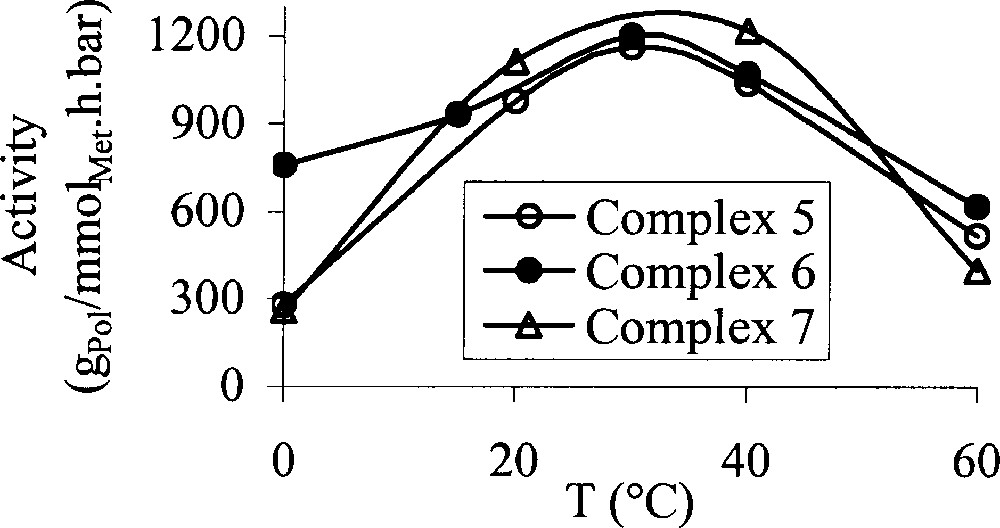
Influence of the polymerisation temperature on the catalytic activity (P = 1 bar; activator: MAO {Al/Met = 400}; solvent: toluene; t = 30 min).
On a second stage, the polymerisation of 1-hexene, as well as its copolymerisation with ethylene, has been examined. First, the homopolymerisation was investigated. Unfortunately, whatever the polymerisation conditions and the catalyst, no polymer was obtained. At this stage, it was difficult to say if this was due to an extremely slow propagation rate or to a poisoning of the catalytic sites by the monomer.
The copolymerisation of ethylene and 1-hexene has then been studied. Whatever the initial amount of 1-hexene, copolymerisation was possible. This is a good proof that this monomer is not a poison for the catalytic sites. The evolution of the catalytic activity versus the initial amount of 1-hexene is displayed in Fig. 3. For the cobalt catalyst (complex 2), no influence of the initial 1-hexene feed has been observed. On the contrary, for the iron catalyst (complex 1), for the highest initial feed, an important decrease of the catalytic activity has been observed.
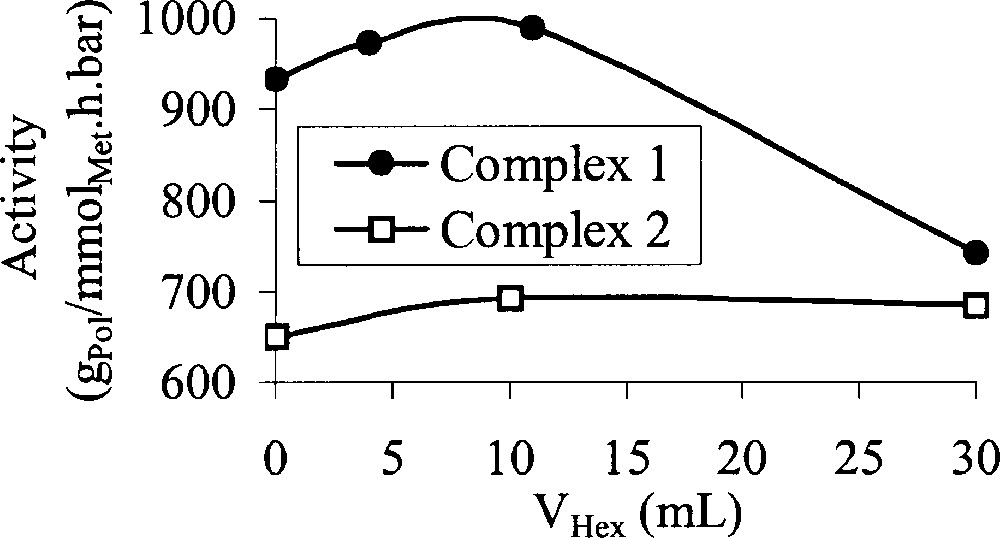
Influence of the initial feed of 1-hexene on the catalytic activity (T = 20 °C; P = 1 bar; activator: MAO (Al/Met = 400); solvent: toluene; t = 30 min).
The second step was to characterize these polymers in order to confirm the incorporation of 1-hexene. The polymers were characterized by Infrared Spectroscopy. Unfortunately, no difference between the polyethylenes and the potential copolymers has been detected with this technique.
The thermal properties have been investigated by DSC. The evolution of the cristallinity with the initial volume of 1-hexene is displayed in Fig. 4. With the cobalt catalyst, the cristallinity is independent of the initial feed. On the contrary, with the iron catalyst, the cristallinity decreases with the initial volume. These first results are in agreement with the incorporation of 1-hexene with the iron catalyst, whereas there is no incorporation with the cobalt catalyst.

Evolution of the cristallinity of ethylene–hexene copolymers with the initial hexene volume.
In order to confirm this hypothesis, high temperature 13C NMR spectroscopy has been performed. The NMR spectra of a polyethylene and a copolymer are presented in Fig. 5. The spectrum of the polyethylene (Fig. 5a) is in good agreement with a highly linear polyethylene. Indeed, the signals detected at 13.5, 22.3 and 31.7 ppm are only due to chain ends. Their relatively high intensity is due to low molar masses. On the spectrum of the copolymer, the signals due to chain ends are still present, but several other peaks have appeared due to the presence of side branches. A statistical analysis has given a number of 15 –CH3 per 1000 C, which would correspond to ∼3.5 mol% incorporation of 1-hexene. But the peak at 13.5 ppm represents both the methyl groups from the n-butyl substituents and the chain ends. The quantity of 1-hexene is thus probably below 3.5 mol%. As a matter of fact, it has been shown successfully that with iron catalysts, 1-hexene is incorporated into polyethylene chains, leading to actual copolymers.

13C NMR spectra of a polyethylene (a) and an ethylene–hexene copolymer (b) synthesized with complex 1.
3 Conclusions
On the one hand, it has been shown that it is still possible to improve the catalytic activity of existing pyridine bis(imine) late transition metal complexes, just by tuning the ligand (especially in the case of cobalt-based catalysts). On the other hand, copolymers of ethylene and 1-hexene have been successfully obtained for the first time, which is a very important result. Indeed, it gives access to Linear Low Density PolyEthylene (LLDPE) with exceptionally high catalytic activity (higher than existing metallocene catalysts). Further work is under progress: kinetic studies as well as other modifications of the ligands.
4 Experimental part
4.1 General procedures and materials
Methylaluminoxane (10%-wt in toluene), 2,6-diacetylpyridine, substituted anilines (2,6-dimethyl aniline; 2-t-butyl aniline; 4-bromo-2,6-dimethyl aniline) and metal chlorides (FeCl2·4 H2O and CoCl2·6 H2O) were purchased from Aldrich and used as received. 2-Chloro-4,6-dimethyl aniline (Aldrich) was distilled before use. Solvents were purified according standards techniques. NMR spectrum of the ligands were obtained on a Brüker AC 200 (200 MHz) at room temperature in CDCl3. High Temperature NMR measurements were performed at 140 °C in 1,3,5-trichlorobenzene-d3 on a Brüker 400 MHz apparatus. Thermal properties were measured on a Perkin Elmer DSC7 apparatus at a heating rate of 10 °C min–1.
4.2 Complex synthesis
All ligands and complexes were synthesized according the following procedures. Except complex 7, all the complexes are very stable.
4.2.1 Step 1: ligand synthesis
4.2.1.1 Synthesis of the 2,6-bis-〚1-(2,6-dimethylphenyl imino) ethyl〛pyridine
In a round-bottomed flask containing 30 ml of methanol, 1 g (6.1 mmol) of 2,6-diacetyl pyridine, 1.77 g (20% in excess) of 2,6-dimethyl aniline and five drops of formic acid were added. The reaction was run at 50 °C during 24 h. By lowering the temperature, the ligand was recrystallized and after filtration and drying, yellow crystals were recovered (1.45 g, 64%). 1H NMR, δ (ppm): 8.50 (d, 2H, Py–Hm), 7.93 (t, 1H, Py–Hp), 7.10 (d, 4H, Ar–Hm), 6.96 (t, 2H, Ar–Hp), 2.25 (s, 6H, N=CMe), 2.07 (s, 12H, Ar–Me). Analysis (C25H27N3) calculated: C, 81.26; H, 7.37; N, 11.37. Found: C, 80.91; H, 7.33; N, 11.38.
4.2.1.2 Synthesis of the 2,6-bis-〚1-(2-t-butylphenyl imino) ethyl〛pyridine
In a round-bottomed flask containing 25 ml of dichloromethane, 1 g (6.1 mmol) of 2,6-diacetyl pyridine, 2.18 g (20% in excess) of 2-t-butyl aniline, 3 g of sodium sulfate and five drops of formic acid were added. The reaction was run at room temperature during 48 h. Sodium sulfate was filtered, the solvent was evaporated and yellow crystals were recovered by recrystallisation from a mixture of methanol/dichloromethane (50:50, v/v) at low temperature (2.36 g, 91%). 1H NMR, δ (ppm): 8.40 (d, 2H, Py–Hm), 7.90 (t, 1H, Py–Hp), 7.45 (d, 2H, NCC(tBu)CHm), 7.20 (t, 2H, NCC(H)CHm), 7.10 (t, 2H, Ar–Hp), 6.57 (t, 2H, Ar–Ho), 2.42 (s, 6H, N=CMe), 1.38 (s, 18H, tBu). Analysis (C29H35N3) calculated: C, 81.84; H, 8.29; N, 9.87. Found: C, 81.40; H, 8.31; N, 9.77.
4.2.1.3 Synthesis of the 2,6-bis-〚1-(4-bromo-2,6-dimethylphenyl imino) ethyl〛pyridine
In a round-bottomed flask containing 30 ml of methanol, 1 g (6.1 mmol) of 2,6-diacetyl pyridine, 2.94 g (20% in excess) of 4-bromo-2,6-dimethyl aniline and five drops of formic acid were added. The reaction was run at 50 °C during 24 h. By lowering the temperature, the ligand was recrystallized and after filtration and drying, yellow crystals were recovered (1.01 g, 31%). 1H NMR, δ (ppm): 8.50 (d, 2H, Py–Hm), 7.97 (t, 1H, Py–Hp), 7.29 (d, 4H, Ar–Hm), 2.28 (s, 6H, N=CMe), 2.07 (s, 12H, Ar–Me). Analysis (C25H25N3Br2) calculated: C, 56.95; H, 4.78; N, 7.97, Br, 30.31. Found: C, 56.95; H, 4.90; N, 7.76; Br, 27.73.
4.2.1.4 Synthesis of the 2,6-bis-〚1-(2-chloro-4,6-dimethylphenyl imino) ethyl〛pyridine
In a round-bottomed flask containing 25 ml of dichloromethane, 1 g (6.1 mmol) of 2,6-diacetyl pyridine, 2.27 g (20% in excess) of 2-chloro-4,6-dimethyl aniline and five drops of formic acid were added. The reaction was run at 50 °C during 72 h. By lowering the temperature, the ligand was recrystallized and after filtration and drying, yellow crystals were recovered (0.88 g, 33%). 1H NMR, δ (ppm): 8.40 (d, 2H, Py–Hm), 7.90 (t, 1H, Py–Hp), 7.45 (d, 2H, NCC(tBu)CHm), 7.20 (t, 2H, NCC(H)CHm), 7.10 (t, 2H, Ar–Hp), 6.57 (t, 2H, Ar–Ho), 2.42 (s, 6H, N=CMe), 1.38 (s, 18H, tBu). Analysis (C29H35N3) calculated: C, 81.84; H, 8.29; N, 9.87. Found: C, 81.40; H, 8.31; N, 9.77.
4.2.2 Step 2: complex synthesis
The general procedure is the following. In a round-bottomed flask, containing 20 ml of THF, the ligand (0.4 g, 1.05 equiv) and the metal chloride were added under argon. A precipitate was rapidly formed (blue for the iron complexes, green for the cobalt complexes). The reaction was run at room temperature during 24 h. At the end, 20 ml of diethyl ether were added. The solution was filtered. The powder was washed three times with diethyl ether, two times with pentane and dried under vacuum.
Complex 1 (yield: 68%). Analysis (C25H27N3FeCl2) calculated: C, 60.51; H, 5.48; N, 8.47; Cl, 14.29. Found: C, 58.69; H, 5.55; N, 7.84; Cl, 13.55.
Complex 2 (yield: 100%). Analysis (C25H27N3CoCl2) calculated: C, 60.13; H, 5.45; N, 8.41; Cl, 14.20. Found: C, 59.38; H, 5.50; N, 8.09; Cl, 13.67.
Complex 3 (yield: 46%). Analysis (C29H35N3FeCl2) calculated: C, 63.06; H, 6.39; N, 7.61; Cl, 12.84. Found: C, 65.59; H, 6.23; N, 6.86; Cl, 12.25.
Complex 4 (yield: 88%). Analysis (C29H35N3CoCl2) calculated: C, 62.71; H, 6.35; N, 7.56; Cl, 12.77. Found: C, 62.07; H, 6.32; N, 7.58; Cl, 12.11.
Complex 5 (yield: 91%). Analysis (C25H25N3FeCl2) calculated: C, 45.91; H, 3.85; N, 6.42; Cl, 10.84; Br, 24.43. Found: C, 46.51; H, 4.09; N, 6.07; Cl, 10.32; Br, 22.83.
Complex 6 (yield: 88%). Analysis (C25H25N3CoCl2) calculated: C, 45.69; H, 3.83; N, 6.39; Cl, 10.79; Br, 24.32. Found: C, 45.80; H, 4.06; N, 5.93; Cl, 9.79; Br, 21.75.
Complex 7 (yield: 52%). Analysis (C25H25N3FeCl4) calculated: C, 53.13; H, 4.46; N, 7.44; Cl, 25.09. Found: C, 53.70; H, 5.13; N, 6.41; Cl, 21.61.
Complex 8 (Yield: 97%). Analysis (C25H25N3CoCl4) calculated: C, 52.84; H, 4.43; N, 7.39; Cl, 24.96. Found: C, 54.37; H, 5.14; N, 6.47; Cl, 21.65.
4.3 Polymerisation procedure
The polymerisations were carried out in a miniclave Büchi (200 ml). A typical procedure for the polymerisation of ethylene with these complexes is given: a Schlenk was charged with 10 μmol of the required complex and 10 ml of toluene. The former solution as well as 20 ml of toluene (and the comonomer if necessary) was injected into the miniclave. The MAO is added last. The polymerisation was let run 30 min and then 30 ml of acidified ethanol was added. The polymer was then precipitated in ethanol and dried under vacuum overnight.
Acknowledgements
The authors acknowledge the ‘Max-Planck-Institut’ in Mainz for high temperature SEC measurements, Bernard Meurer for high temperature NMR measurements, Suzanne Zehnacker for DSC measurements and the ‘Centre National de la Recherche Scientifique’ (CNRS) for financial support.



