1 Introduction
Chemical elements naturally occur in the Earth's crust. However, human activities have introduced high loads of these constituents in the environment, making it now sometimes difficult to differentiate between natural and anthropic contributions.
Trace metals (TM) such as cadmium (Cd) and lead (Pb) are considered among the most critical pollutants in natural environments and for human life (Manahan, 2000) due to their toxicity, persistence, and bio-accumulation capacity at different levels of the food chain.
Trace metals are ubiquitous in the global environment (Benguedda et al., 2011; Bilos et al., 2001; Hamdoun et al., 2015; Hlavay et al., 2001; Hosono et al., 2010; Kouidri et al., 2016; Okbah et al., 2014; Rahman et al., 2014; see also the references in Chabaux et al., 2015). In natural aquatic ecosystems, TM mostly occur at low concentrations. However, in recent times, there have been rising problems with increasing contamination levels of heavy metals in those environments and pollution of aquatic habitats. Sediments in estuarine environments or on the continental shelf usually act as a sink for land-derived aquatic pollutants, and especially for trace metals (Tang et al., 2010). Consequently, sediment-associated contaminants can further influence the concentrations of TM in both the water column and biota if they are desorbed or become available to benthic organisms (Díaz-de Alba et al., 2011). Therefore, sediments often served as indicators of the burden of heavy metals in coastal environments as they are ultimately stored in this material (Gargouri et al., 2011).
In some works, chemical analyses in vertical sediment profiles are used to reconstruct the contamination history of aquatic systems (Evenset et al., 2007; Lepland et al., 2010; Lourino-Cabana et al., 2011). The present study aims to determine the levels of major (Al, Fe, Mg, Ti) and trace elements concentrations (V, Cr, Mn, Co, Ni, Cu, Zn, Mo, Cd, and Pb) in sediments from the coastal areas of the Ghazaouet Bay (northwestern Algeria). This area has been identified as a hot spot of coastal anthropization due to important industrial activities (Belhadj, 2008). Thus, it is now crucial to evaluate the importance of pollution according to objective criteria such as EF or Igeo. Three selected sites were chosen along the near coast to elucidate the relationship between the location of the industrial plant of zinc electrolysis as well as other polluting sources in the Ghazaouet area and the resulting heavy metal contamination in sediments.
2 Materials and methods
2.1 Study area
The Ghazaouet Bay is located in northwestern Algeria (N 37°25′19″, W 122°05′06″) at approximately 10 km from the Moroccan border (Fig. 1). The Ghazaouet town hosts an important harbor on the coast for the entire northwestern region of Algeria. Coastal seawaters are continuously exposed to industrial, urban, and agricultural wastes, releasing huge quantities of contaminants and trace metals, especially Zn and Cd, originating from a large industrial complex of zinc electrolysis, near the harbor of Ghazaouet (Benguedda et al., 2011). Moreover, tailings disposal or residues from ore exploitation by the industry occur outdoors as cliffs and are submitted to the leaching of meteoric waters that further reach the shore.
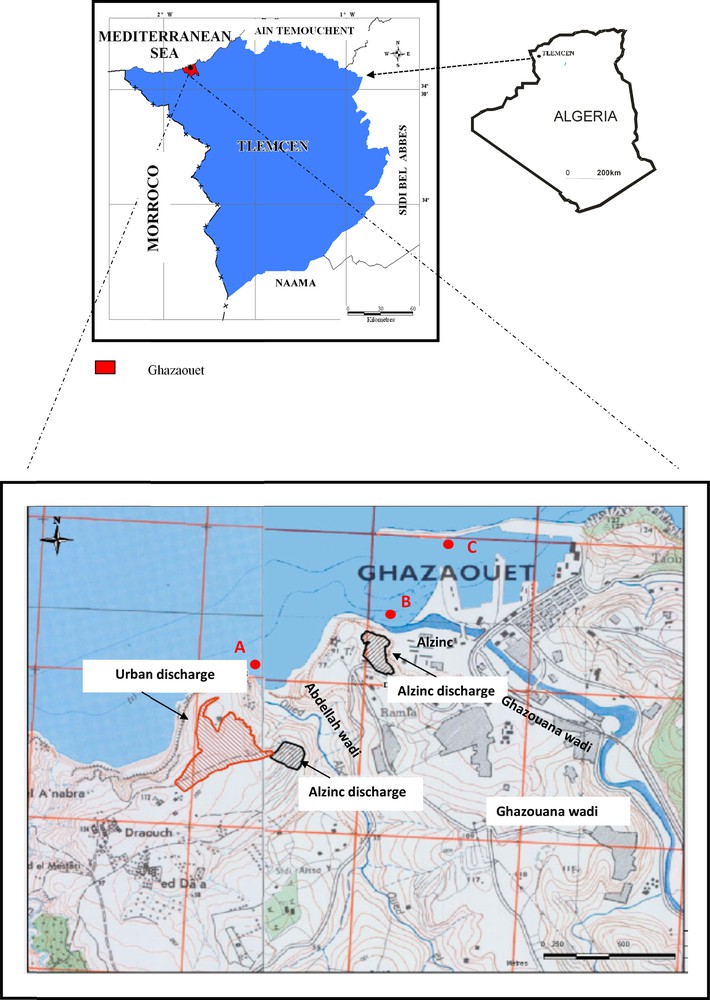
Map of the studied area showing the Ghazaouet area (A: Beach of Wadi Abdella, B: Beach of Wadi Ghazouana and C: Harbor of Ghazaouet), where the samples were collected.
2.2 Sediment sampling
Sampling was carried out monthly between July 2010 and June 2011. Three stations were investigated in this area: the first on the beach of Wadi Abdella (A) located west of Ghazaouet. The second station is situated on the beach of Wadi Ghazouana (B) 1 km east from the first station. The third one is located inside the harbor of Ghazaouet (C), 500 m east from the Ghazaouet town (Fig. 1). Surface sediment samples were taken in shallow water. Cores are 5 cm in depth. Sediments were collected by diving in harbor “C” and by scraping surface sediments using a plastic shovel in stations “A” and “B”. After collection, the samples were placed in polyethylene bags and transported to the laboratory in iceboxes. Thirty-five sediment samples have been collected for analysis.
2.3 Sediment geochemistry analysis
Sediment samples were air-dried for one week, and after performing grain size studies the finest fraction (< 63 μm) was separated. A mixture of acids (HF, HCl, HNO3) in proportion 1:3:1 (v:v:v), was used for the sediment digestion in a microwave oven (Anton Paar Multiwave 3000). Acids exhibiting high purity were employed. Extracts were then evaporated to dryness and diluted afterwards with Milli-Q water. The resulting solutions were stored at 4 °C until ICP-MS quantitative analysis of trace elements (Al, Fe, Mg, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Mo, Cd, and Pb) using an Agilent 7700X instrument. The accuracy of the analytical procedures for the determination of total metal concentrations was checked using the NIST 1646a estuarine sediment standard. These were analyzed under the same experimental conditions. Two replicate analyses of this reference material processed in the same time as samples are in good accordance with certified values (Supplementary material, Table S1).
2.4 Geo-accumulation index (Igeo)
According to Müller (1969), the geo-accumulation index (Igeo), highlights the assessment of sediment and soil pollution by heavy metals (e.g., Amin et al., 2009; Singh et al., 2005; Williams and Block, 2015). The values of the geo-accumulation index can be calculated according to Müller (1969) as follows:
| (1) |
where Cn is the measured concentration of the examined metal (n) in the examined bottom sediment, and Bn the geochemical background value of element n in the surrounding rocks or, if not available, in the average shale (e.g., Dali-youcef et al., 2005; Turekian and Wedepohl, 1961); 1.5 is the correction factor addressed due to lithogenic effects (Lin et al., 2008).
2.5 Enrichment factor (EF)
It is common to estimate the anthropogenic impact on coastal sediments via the calculation of trace element enrichment factors (EF) (e.g., Kouidri et al., 2016; Lourino-Cabana et al., 2011; Rahman et al., 2014; Rodriguez-Barroso et al., 2010). They result from a twofold normalization of the analyzed samples with reference materials that refer to uncontaminated background levels (Dickinson et al., 1996; Salomons and Förstner, 1984) – see Eq. (2). Because TM are generally associated with the finest particles, TM concentrations must be divided first by the concentrations of an element (X) in the samples that is of natural origin and that is highly associated with the grain size fraction of clays. Then, a second normalization with a reference background sample is applied. It reflects natural TM concentrations that are commonly recorded in the environment (soils or surrounding rocks).
| (2) |
Theoretically, if the EF is higher than one, it may be indicative that the sediment is mainly originating from an anthropogenic source and can be used for assessing the degree of pollution. In fact, it is more commonly admitted that EF > 2 reflects enrichment from anthropogenic sources (Sutherland, 2000). If natural background concentrations can be determined regionally (as recommended by Rubio et al., 2000), this may be already the case for EF > 1.5 (Bhuiyan et al., 2010). In our case, the background concentrations of V, Cr, Mn, Ni, Cu, Zn, and Pb refer to the local background from Dali-youcef et al. (2005), and the background concentrations of Mo, Co and Cd refer to the mean composition of the upper continental crust and were taken from Turekian and Wedepohl (1961).
Several lithogenic elements have been used as reference elements because they are considered to be geochemically stable, hosted by resistant minerals, and conservative in most geochemical environments. The used element in this study is Ti for EF calculation (Boës et al., 2011) because Ti has relatively high natural concentration, and is therefore not expected to be substantially enriched from anthropic sources in Ghazaouet Bay. The metal to Ti ratio is relatively constant in the crust. It is an abundant metal and the Ti concentrations are not likely to be significantly affected by anthropogenic sources (Dali-youcef et al., 2005). Other elements such as Al and Fe have been also used as references (e.g., Bhuiyan et al., 2010; Daskalakis and O’Connor, 2005; Dumas et al., 2015) for marine sediments.
2.6 Statistical methods
The statistical significance was computed using ANOVA1. A probability of 0.05 or less is considered as significant. Factor analysis based on principal component analysis (PCA/FA) was used to ascertain sources of contamination (natural and anthropogenic). Pearson's correlation was used to identify the interrelationships between metals and other parameters and to support the results obtained by PCA/FA. All calculations were performed using the statistical package Minitab 16.
3 Results and discussion
3.1 Distribution of major and minor metals
From the total weight of each sample and the weight of its fraction < of 63 μm, we have determined the percentage of each fraction smaller than 63 μm. The obtained results indicate that 5% of particles of stations “A” and “B” and 42% of particles of station “C” exhibit diameters smaller than 63 μm. This fraction was chosen for our analysis (Salomons and Förstner, 1984).
Supplementary material, Table S2 exhibits minimum–maximum and average concentrations of major elements (mg/g dry weight) and trace elements (mg/kg dry weight). The variability on these averages arises from the temporal variability on more than a dozen of samples in surface sediments of the Ghazaouet Bay stations (A: beach of Wadi Abdellah, B: beach of Wadi Ghazouana, and C: harbor of Ghazaouet). The average shale data given by Dali-youcef et al. (2005), and the background concentrations of Mo, Co, and Cd refer to the mean composition of the upper continental crust taken from Turekian and Wedepohl (1961), were used as background data.
According to the results, the aluminum average concentrations were 37.60 mg/g (12.47–60.38 mg/g) at station “A”, 36.68 mg/g (13.92–56.02 mg/g) at station “B” and 50.96 mg/g (23.72–60.07 mg/g) at station “C”. Iron average concentrations were 32.05 mg/g (11.62–44.07 mg/g) at station “A”, 34 mg/g (14.52–52.9 mg/g) at station “B” and 35.77 mg/g (20.98–38.17 mg/g) at station “C”.
Magnesium is a lithophile metallic element and a major constituent of many mineral groups, including carbonates. The average concentrations were 17.42 mg/g (5.80–24.57 mg/g), 14.61 mg/g (5.88–26.88 mg/g), and 11.14 mg/g (4.07–13.13 mg/g) at stations “A”, “B” and “C”, respectively. In northeastern Algeria, large dolomite formations outcrops (Thinton, 1941) are responsible for the high Mg concentrations in sediments. The titanium concentration levels depend on many parameters in relation with the constitution of the area (Benest and Elmi, 1978; Thinton, 1941). The average concentrations were 5.39 mg/g (1.88–7.16 mg/g), 5.49 mg/g (2.12–8.06 mg/g) and 4.28 mg/g (4.15–4.39 mg/g) at study stations, respectively. In the present study, Al, Fe Mg, and Ti concentrations are in the range of the background data (31 mg/g for Al, 33 mg/g for Fe, 10.8 mg/g for Mg and 3.5 mg/g for Ti) (Dali-youcef et al., 2005).
The average concentrations of vanadium, manganese, cobalt and molybdenum were 100.40 mg/kg (35.76–135.60 mg/kg), 546.80 mg/kg (247–787 mg/kg), 14.93 mg/kg (6.50–21.54 mg/kg) and 1.04 mg/kg (0.45–2.41 mg/kg) at station “A” respectively, 94.26 mg/kg (38.76–148.19 mg/kg), 573 mg/kg (232–859 mg/kg), 15.05 mg/kg (6.55–23.95 mg/kg), and 0.95 mg/kg (0.42–1.44 mg/kg) at station “B”, respectively, and 101.35 mg/kg (43.5–110.5 mg/kg), 351.26 mg/kg (243–399 mg/kg), 10.43 mg/kg (4.77–11.16 mg/kg) and 2 mg/kg (1.50–3.65 mg/kg) at station “C”, respectively. These concentrations are in the range of the background data (V: 73 mg/kg, Mn: 391 mg/kg, Co: 19 mg/kg, and Mo: 2.6 mg/kg) (Dali-youcef et al., 2005; Turekian and Wedepohl, 1961) (Supplementary material, Table S2).
The average concentrations of chromium were 115.93 mg/kg (57.08–186.11 mg/kg), 126.63 mg/kg (62–320 mg/kg) and 81.72 mg/kg (38–103 mg/kg), and those of nickel were 43.53 mg/kg (19.73–61.69 mg/kg), 44.63 mg/kg (20.04–76.63 mg/kg), and 36.98 mg/kg (26.43–40.68 mg/kg) at all stations, respectively. These concentrations are found slightly higher than the local background (Cr: 55 mg/kg and Ni: 20 mg/kg) (Dali-youcef et al., 2005) (Supplementary material, Table S2).
The results for copper, zinc, cadmium, and lead indicate high concentrations, easily exceeding the average reference levels in all stations (Supplementary material, Table S2), (Cu: 23 mg/kg; Zn: 57 mg/kg; Cd: 0.3 mg/kg; Pb: 13 mg/kg) (Dali-youcef et al., 2005; Turekian and Wedepohl, 1961). The average concentrations of copper were 63.90 mg/kg (12.46–247.14 mg/kg), 92.15 mg/kg (37.5–153.4 mg/kg), and 210.66 mg/kg (12–521 mg/kg) at the study stations, respectively. For zinc, they were 282 mg/kg (109–486 mg/kg), 1901 mg/kg (624–4376 mg/kg), and 2252 mg/kg (49–2858 mg/kg), for cadmium were 3.87 mg/kg (1.06–9.09 mg/kg), 19.57 mg/kg (5.81–34.69 mg/kg), and 11.50 mg/kg (0.21–15.61 mg/kg) whereas those for lead were 61.38 mg/kg (22–113 mg/kg), 374 mg/kg (288–427 mg/kg), and 356 mg/kg (12–459 mg/kg) at all stations respectively. The highest concentrations were obtained for zinc at station “C”.
The important quantities of zinc and cadmium, in the region of Ghazaouet, come from the discharges of the Metanof (Alzinc) plant, which extracts zinc and cadmium, and manufactures sulfuric acid. Despite the recent installation of a water treatment unit, zinc concentrations are still very high and exceed the average reference level. The important quantities of lead can be probably explained by the industrial releases of waste and many chemicals accompanying the production process. The amount of industrial sewage, emanating from the surrounding town is directly discharged into the harbor “C” and in the neighboring area “B”. These facts may explain the highest levels of Cu and Zn found in surface sediments at station “C”. Cantillo and O’Connor (1992) established the average Cd concentrations in sediments throughout world from 1.5 to 7.7 mg/kg, with maximum values close to industrial complexes and sewage pipelines. Pb in sediments is mainly bounded to fine particles or oxides coatings, and is also essentially associated with organic matter or mineral sulfides in anaerobic conditions.
Results of Supplementary material, Table S2 show that the concentrations for Mg and Co were significantly (P < 0.05) and significantly high for Al, Mn, Cu, Zn, Mo Cd and Pb (P << 0.005) between stations (ANOVA1).
Titanium, element major of aluminosilicates, fulfils perfectly the requirements of a nonreactive, inert standard element with respect to the physicochemical parameters of the environment, and is stable in aquatic environments (Aloupi and Angelidis, 2001; Din, 1992; Rubio et al., 2000). This element is used to assess the mobility of other metals. It is used to normalize major and trace elements in the Ghazaouet sediments, which are shown in Figs. 2, 3a and b). Heavy metals normalized with reference elements can be used to remove the influences of geological and hydrodynamic processes and to estimate the anthropogenic impact (Soto-Jiménez and Páez-Osuna, 2001). Figs. 2, 3a and 3b show the variations of major elements (Al, Fe, and Mg) and trace elements (V, Cr, Mn, Co, Ni, Cu, Zn, Mo, Cd and Pb), respectively, versus the reference element (Ti). The lines in each one of the figures correspond to the slope with the ratio element/Ti defined in the UCC.
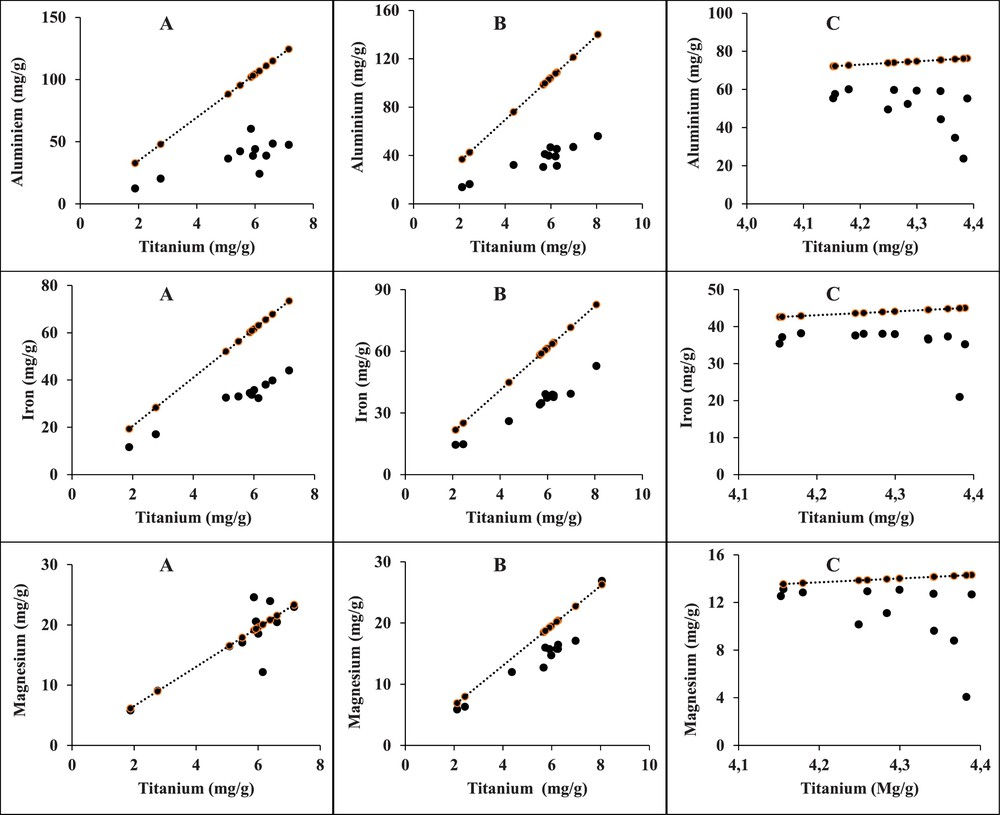
Variations of major element concentrations (Al, Fe and Mg) versus the reference element Ti in surface sediments collected at study stations in the Ghazaouet Bay.
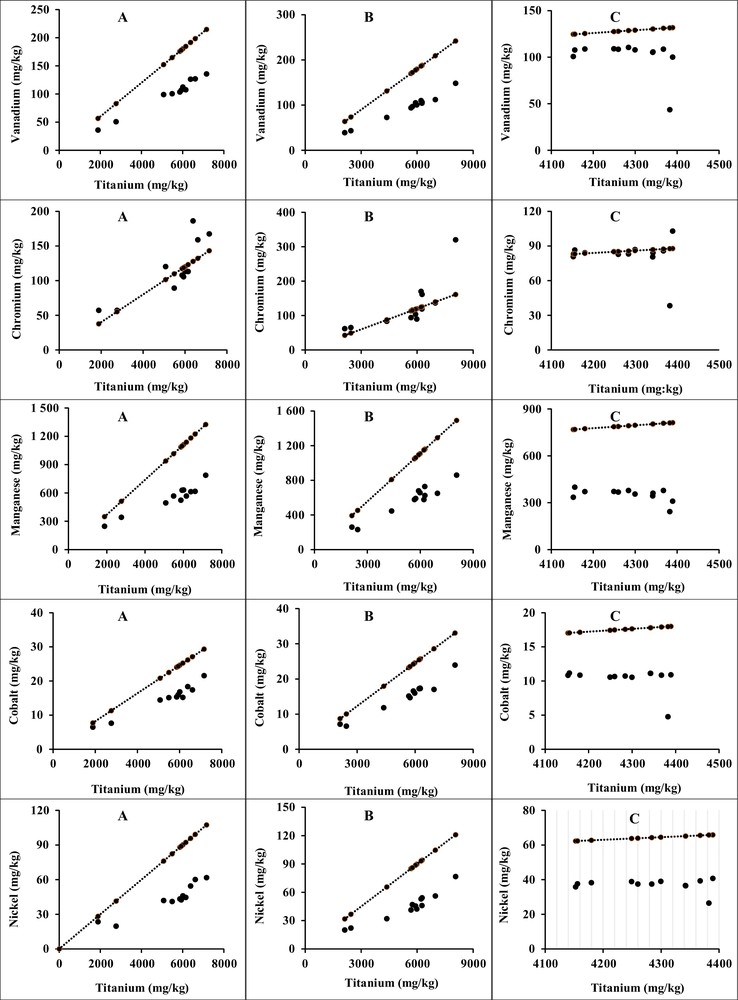

a. Variations of trace element concentrations (V, Cr, Mn, Co and Ni) versus the reference element Ti in surface sediments collected at study stations in the Ghazaouet Bay. b. Variations of trace element concentrations (Cu, Zn, Mo, Cd and Pb) versus the reference element Ti in surface sediments collected at study stations in the Ghazaouet Bay.
Most of normalized elements show significant increases in each one of the sediment profiles. In all stations, the Al–Ti, Fe–Ti and Mg–Ti relationships are linear (Fig. 2). The Al/Ti (4–10), Fe/Ti (5–8) and Mg/Ti (1–4) ratios vary slightly, apart from a few points. It should be noted that the Al/Ti ratio can reach 14.37 in station “C” (harbor), where the emissaries of the Alzinc factory have been found.
The variations of the ratio X/Ti are less important for V (0.01–0.03), Cr (0.01–0.04), Co (0.001–0.003), Ni (0.01) and Mo (0.0002–0.0006) (Fig. 3a, 3b), and are more significant for Cu (0.01–0.12), Zn (0.01–0.76), Cd (0.0001–0.016) and Pb (0.001–0.17) (Fig. 3b). We observe also an enrichment with copper, zinc, cadmium, and lead in Ghazaouet Bay, due mainly to the industrial wastes.
In the case of manganese, we observed important values of the Mn/Ti ratio (0.06–0.13). The reason of these finding is the geochemical nature of the region (Thinton, 1941).
Major elements (Al, Fe, and Mg) and minor elements (V, Cr, Mn, Co, Ni and Mo) show well-defined linear correlations with Ti, which suggests that they are immobile or less mobile during the study period (Figs. 2, 3a, b). In contrast, Cu, Zn, Cd and Pb show a wide range and do not correlate with Ti in sediments (Fig. 3b). Their large variation likely attests to an enrichment by these elements.
3.2 Factor analysis
The results of the PCA are presented in Fig. 4. Two principal components are extracted, which account for 100% of the total variance. The first factor (PC1) explains 74.60% of the total variance, and contains a positive loading of V, Mo, Al, Cu, Fe, Zn, Pb, and Cd and a negative loading of Mg, Co, Ti, Ni, Mn, and Cr. The second factor (PC2) accounts for 25.4% of the total variance (Fig. 4). Having two factors with different loadings means that two different contributions are involved in the determination of major and trace element concentrations in marine sediments (Rahman et al., 2014).
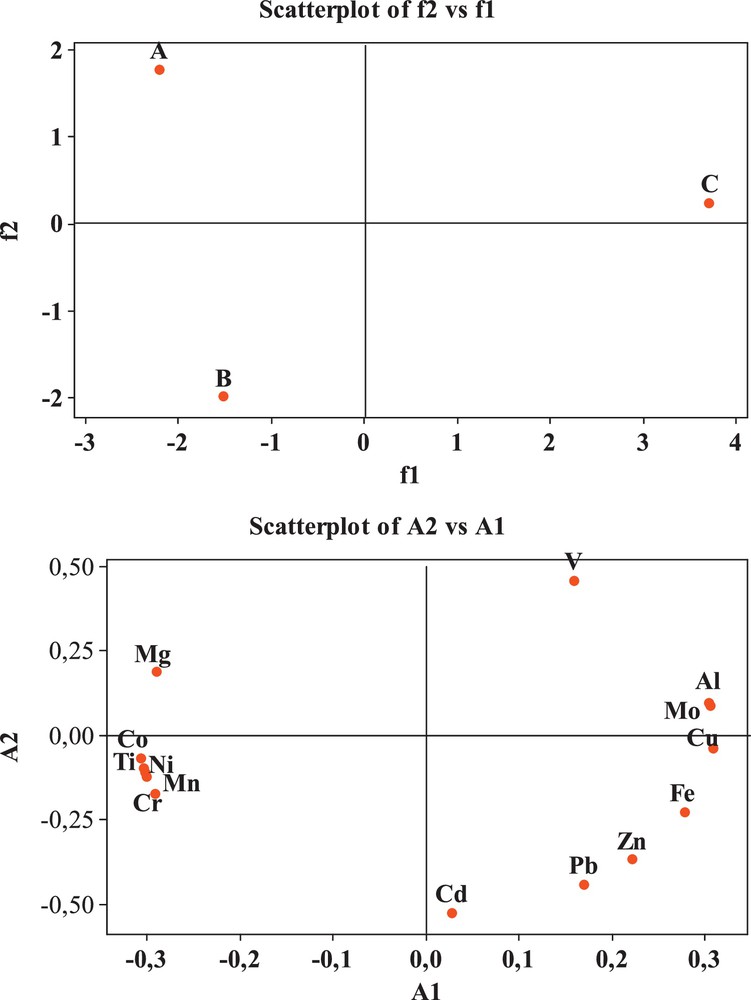
Biplots for first and second axes of the PAC bases on mean values of major elements (Al, Fe, Mg and Ti) and trace elements (V, Cr, Mn, Co, Ni, Cu, Zn, Mn, Cd and Pb) in surface sediments collected at study stations from the Ghazaouet Bay.
Results consistent with the PCA were obtained from CA, where the fourteen metallic elements were grouped into two significant clusters (Fig. 5): the first cluster (C1) contains Al, Mo, Cu, Fe, Zn, Pb, Cd and V (correlated in factor 1 in PCA); the second cluster (C2) contains Mg, Ti, Mn, Ni, Co, and Cr (which was well consistent in factor 2 in PCA). At a higher distance, the two clusters (C1 and C2) are merged to form cluster (C3).

Dendrogram derived from the hierarchical cluster analysis of major and ETM contents in the analyzed sediments.
A correlation matrix was calculated for major and trace elements to establish relationship among metals and determine common origin of metals in Ghazaouet Bay sediments. Pearson's correlation matrix for all samples is presented in Supplementary material, Table S3. Strongly positive correlations were observed among the elements in cluster C1; i.e. between Al and Mo (r = 1.000), Al and Cu (r = 0.971), Al and Fe (r = 0.819), Al and Zn (r = 0.592), Al and V (r = 0.648), Mo and Cu (r = 0.967), Mo and Fe (r = 0.809), Mo and Zn (r = 0.578), Mo and V (r = 0,661), Cu and Fe (r = 0.932), Cu and Zn (r = 0.767), Cu and Pb (r = 0.608), Fe and Zn (r = 0.948), Fe and Pb (r = 0.855), Zn and Pb (r = 0.976), Zn and Cd (r = 0.760), and Pb and Cd (r = 0,883). In addition, positively strong correlation were found among the components of cluster C2 (i.e. between Mg and Ti, Mg and Mn, Mg and Ni, Mg and Co, Mg and Cr, Ti and Mn, Ti and Ni, Ti and Co, Ti and Cr, Mn and Ni, Mn and Co, Mn and Cr, Ni and Co, Ni and Cr, Co and Cr with r = 0.856, 0.841, 0.827, 0.884, 0.769, 1.000, 0.999, 0.998, 0.989, 1.000, 0.996, 0.993, 0.994, 0.995, 0.979, respectively (Supplementary material, Table S3). Combined with the above analysis, we can see that the first group (Al, Mo, Cu, Fe, Zn, Pb, Cd and V) mainly came from artificial sources, whereas the second group (Mg, Ti, Mn, Ni, Co, and Cr) mainly came from the natural environment.
3.3 Comparison with other Mediterranean sites
Supplementary material, Table S4 gives the minimum, maximum metal concentrations reported in different Mediterranean coastal sediments including those obtained in this work. Recently published values from Rodriguez-Barroso et al. (2010), Díaz-de Alba et al. (2011), Gargouri et al. (2011), Tessier et al. (2011), Okbah et al. (2014), Dang et al. (2015), Kouidri et al. (2016), Misson et al. (2016) were chosen to highlight some real levels of contamination and compare them with our data, with the recommended values for unpolluted sediments (GESAMP, 1982, Salomons and Förstner, 1984) and with sediment quality guidelines TEC (Threshold Effect concentrations) and PEC (Probable Effect Concentrations) (MacDonald et al., 2000). The minimum and maximum values were considered for each metal studied. The Fe concentrations in Ghazaouet Bay were found higher than in other sites (Gargouri et al., 2011; Kouidri et al., 2016, Misson et al., 2016; Okbah et al., 2014); Mn concentrations were found lower than literature values. Both Fe and Mn were lower than the values for unpolluted sediments (Fe: 41 mg/g, Mn: 770 mg/kg) recommended by Salomons and Förstner (1984).
Chromium concentrations were found higher than those found in Tangiers Bay, Sfax coast and Toulon Bay (Gargouri et al., 2011; Misson et al., 2016; Rodriguez-Barroso et al., 2010), but lower than sediment quality guidelines TEC (Threshold Effect concentrations) and PEC (Probable Effect Concentrations) (MacDonald et al., 2000). Ni concentrations were higher than those found in Toulon Bay (Dang et al., 2015 and Misson et al., 2016) and PEC (Probable Effect Concentrations) (MacDonald et al., 2000). Except for Toulon Bay, Cu and Pb levels were higher in our results than those reported in all references of Supplementary material, Table S4, (Cu: 33 mg/kg; and Pb: 19 mg/kg), recommended by Salomons and Förstner (1984). In general, the degree of marine contamination in Ghazaouet Bay remains relatively high for Cu, Zn, Cd and Pb, compared to the recommended values for unpolluted sediments, TEC (Threshold Effect Concentrations) and PEC (Probable Effect Concentrations) (GESAMP, 1982, MacDonald et al., 2000; Misson et al., 2016; Salomons and Förstner, 1984).
3.4 Geo-accumulation index and enrichment factors
Table S5 (Supplementary material) shows the index values Igeo, and enrichment factors EF at the three stations (A, B, and C). According to Dali-youcef et al. (2005), the local background values (mg/kg dry weight) adopted are fixed at 30,570 for Al, 32,490 for Fe, 73.19 for V, 54.91 for Cr, 391 for Mn, 20.10 for Ni, 22.53 for Cu, 57.24 for Zn, 2.6 for Mo, 0.3 for Cd, 13.27 for Pb, and, according to Turekian and Wedepohl (1961), the background values adopted are 90 for Cr and 0.3 for Cd. The elements Zn, Cd and Pb showed the highest Igeo values at all stations. Igeo for Zn varied between 1.383 and 4.381, for Cd varied between 3.106 and 5.443 and for Pb varied between 1.032 and 3.689. Those values put Zn for both stations “B” and “C” and Cd for the station “C” in class 5, corresponding to highly to very polluted sediments, and Cd for the stations “A” and Pb for both stations “B” and “C” in class 4, which corresponds to highly polluted sediments. The values Igeo in stations “B” put Cd in class 6, corresponding to very highly polluted sediments. The Igeo indexes for both Zn and Pb put this metal in class 2, corresponding to moderately polluted sediments for the stations “A”. The Igeo indexes for Cu varied between 0.869 and 2.590, putting Cu in class 1 for the station “A”, corresponding to unpolluted to moderate polluted, in class 2 for the station “B”, corresponding to moderately polluted, and in class 3 for the station “C” corresponds to moderate to high polluted sediments. The Igeo values in all stations put both Cr and Ni in class 1, which corresponds to unpolluted to moderate polluted sediments. The Igeo indexes for Mn varied between 0.444 and 0.262, putting this metal in class 0, corresponding to unpolluted for the station “C”, and in class 1 for both stations “A” and “B”, corresponding to unpolluted to moderate polluted sediments. The values for Al, Fe, V, Cr, Co, and Mo were found to be lower than 0, indicating unpolluted sediments (class 0) (Müller, 1969).
Metal accumulation in sediments is related to different parameters. Also, the results show that no enrichment was observed for Co and Mo at all stations, Al, Fe, and V at both stations “A” and “B”, and Mn at station “C” (EF < 1). The enrichment was less for Cr and Ni at all stations, Al, Fe, and V at stations “C”, Mn and Cu at both stations “A” and “B” and Pb at station “A” (1 < EF < 3). The enrichment was moderately severe for Cu at station “C” and Cd at station “A” (5 < EF < 10), and more severe for Zn at station “B” and Pb at both stations “B” and “C” (10 < EF < 25). The enrichment became very severe for Zn at station “C” and Cd at both stations “B” and “C” (25 < EF < 50) (Supplementary material, Table S5), which receive agricultural and industrial domestic wastes.
4 Conclusion
This investigation consisted in a detailed analysis of the concentrations of major and trace elements in sediments in Ghazaouet Bay (northern Algerian coast). The metal concentrations measured in sediments indicate that the area of Ghazaouet is severely polluted. A high variability of metal accumulations was found at the three stations under study. Based on the results obtained, the sediments from Ghazaouet Bay show that the average concentrations of copper, zinc, cadmium, and lead exceed the background values. The impact of anthropogenic heavy metal pollution in Ghazaouet Bay was evaluated using the geo-accumulation index (Igeo) and the enrichment factor (EF) at all stations. The geo-accumulation indices (Igeo) are clearly variable and suggest that sediments were moderately to highly polluted for Cu, and highly to very polluted for Zn, Cd, and Pb. The results of normalized enrichment factors (EF) show higher values for Cu (1.79–7.42), Zn (2.55–25.66), Cd (8.43–41.72), and Pb (2.07–15.11), thus confirming a case of pollution. Compared with the sediment quality guidelines, in sediment samples Cu, Zn, Cd and Pb levels were above the TEC values, showing that contaminated sediments at Ghazaouet Bay can affect adversely sediment-dwelling organisms. Consequently, continuous monitoring and efforts of remediation might be required to improve the coastal environment near the industrialized area.
Acknowledgements
The authors would like to express sincere gratitude to Samia Khoudir, mounia Al-Hassani, Bruno Charrière and Christophe Menniti for their help.


