1 Introduction
Urease (EC 3.5.1.5) is an enzyme that catalyzes the conversion of urea into ammonia and carbon dioxide [1,2]. While urea amidolyase breaks down urea in two steps, urease does this in a single step [3]. Urease can be found in a great variety of algae, bacteria, plants, and fungi [1,4,5]. Although, the structure, type and number of subunits, their molecular weight and the amino acid sequence of the ureases differ, the amino acid sequence of the active sites are conserved and therefore, the mechanism of the enzyme activity is the same for all ureases [5,6]. Urease isolated from the plant jack bean is a homohexameric molecule whose active site contains two nickel ions that are involved in binding of substrates as well as of some of the inhibitors [7,8]. Each jack bean urease subunit possesses 15 cysteine residues, which makes urease a thiol-rich enzyme [6,9]. However, without denaturation of the enzyme, only six of the 15 cysteine residues are accessible for reagents. One of these residues, cysteine-592, is recognized to play a critical role in the catalytic activity of the enzyme [10,11]. Ureases are ubiquitous in nature and it is known that they are directly associated with the formation of infection stones and contribute to the pathogenesis of several infectious diseases like e.g. pyelonephritis, an ascending urinary tract infection [12]. Additionally, they are an important factor in the pathogenesis of many clinical conditions [13,14]. Helicobacter pylori (H. pylori) produces high quantities of urease and thereby large amounts of ammonia are formed which makes it possible for the bacteria to inhabit the stomach [12]. This high amount of ammonia in the stomach is now accepted as the major cause of peptic ulcers [2,15]. For this reason, urease inhibitors have attracted a lot of attention as potent anti-ulcer drugs [16]. Due to the diverse function of the enzyme, the study of urease inhibition not only has medical but also environmental and agronomic significance [6]. The development of potent and specific inhibitors could lead to the treatment of infections caused by urease-producing bacteria [17]. In 2003, Kawase et al. reported for the first time that several α,β-unsaturated ketones are inhibitors for jack bean urease. The most potent α,β-unsaturated ketones were cyclic and of low-molecular weight, e.g. 2-cyloheptene-1-one (IC50 = 0.16 mM), 2-cyclohexene-1-one (IC50 = 0.69 mM), 2-cyclopentene-1-one (IC50 = 0.97 mM) [12].
To the best of our knowledge, no further report on other α,β-unsaturated carbonyl compounds as potential inhibitors of urease has been published. Ethacrynic acid, a loop or high ceiling diuretic, possesses an α,β-unsaturated carbonyl unit and is used to treat high blood pressure and edema [18,19]. We have recently synthesized several analogues of ethacrynic acid lacking the α,β-unsaturated carbonyl unit and discovered that some of our analogues possess a significant potency to inhibit the migration of several cancer cell lines [20,21]. We herein report the inhibitory effect of ethacrynic acid (IV-1) and seven of its analogues (IV-2-8) (Fig. 1) on jack bean urease.

Ethacrynic acid (IV-1) and seven of its analogues (IV-2-8). Compounds IV-1 and IV-2, IV-3 and IV-4, IV-5 and IV-6, as well as IV-7 and IV-8 are considered as chain length analogues.
2 Experimental
2.1 General procedure for the preparation of ethacrynic acid and its analogues
The synthesis of ethacrynic acid (IV-1) and its seven analogues (IV-2-8, Fig. 1) was accomplished by a three-step reaction shown in Scheme 1. The Friedel–Crafts acylation reaction of the phenol or the substituted phenols I-1-4 (Scheme 1), respectively, with propanoyl chloride (n = 1) or butanoyl chloride (n = 2), respectively, was performed in the presence of powdered aluminium chloride (AlCl3) in carbon disulfide, as recently described by us [22]. Compounds II-1-8 were purified by flash column chromatography on silica gel and consecutively refluxed in acetone for 48 hours in the presence of 1.2 equivalents of ethyl bromoacetate and two equivalents potassium carbonate (K2CO3) to yield compounds III-1-8. In the third step, an aldol condensation reaction, compounds III-1-8 were refluxed in an ethanol/water (50/50) mixture for 24 hours in the presence of two equivalents of formaldehyde and 2.5 equivalents of K2CO3. Compounds IV-1-8 were obtained by flash chromatography using a hexanes/ethyl acetate/methanol mixture as the eluent.
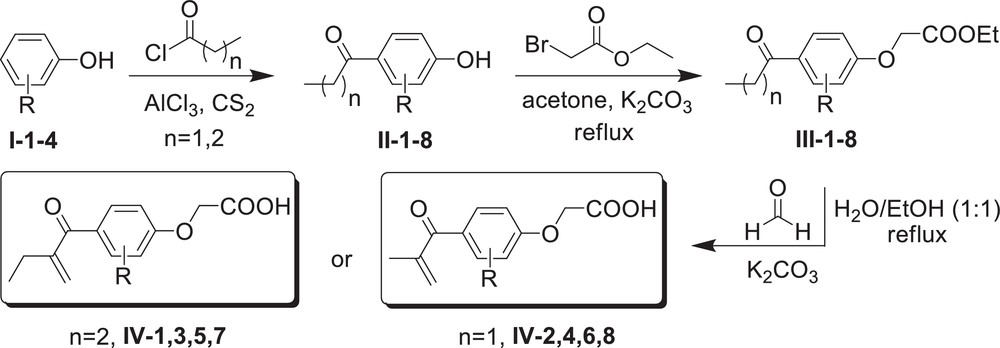
Synthesis of ethacrynic acid (IV-1) and seven of its analogues (IV-2-8).
2.2 Urease inhibition assay
The assay mixture containing 50 μL jack bean urease (5 mg of type III urease dissolved in 0.5 mL phosphate buffered saline [PBS]) and 1 μL of the test compound (dissolved in dimethyl sulfoxide [DMSO]) was preincubated for 60, 120, 180, and 240 minutes at room temperature in a 96-well assay plate. After preincubation, 150 μL urea broth containing phenol red pH indicator (9.68 g urea medium dissolved in 250 mL reverse osmosis [RO] water) was added to each well. Once the substrate and the indicator were added, a micro plate reader was used to measure the initial absorbance for each well at 595 nm. The results (change in absorbance per min) were processed using SoftMax Pro software (Molecular Devices). Inhibition percentages were calculated using the formula 100–(ODtestwell/ODcontrol) × 100. In order to determine IC50 values, inhibition studies were carried out at various concentrations of the test compound (1.0 mM, 0.5 mM, 0.4 mM, 0.3 mM, 0.2 mM, and 0.1 mM). All reactions were performed in triplicate.
3 Results and discussion
Compounds IV-1-8 (Fig. 1) all of which possess an α,β-unsaturated carbonyl unit were subsequently tested against jack bean urease. We initially tested the inhibitory effects of our eight compounds (IV-1-8) at various concentrations thereby keeping the concentration of urease constant. Before adding the urea broth, we preincubated the enzyme with the respective compound for 60 minutes. The results of our enzyme assays are shown in Table 1. Ethacrynic acid (IV-1) itself inhibits jack bean urease by 28% (0.5 mM) or 43% (1.0 mM), respectively (IC50 = 1.21 mM). Similar observations were made for its chain length analogue (IV-2) whose inhibitory activity of 26% (0.5 mM) or 41% (1.0 mM), respectively (IC50 = 1.32 mM), is slightly lower than that observed for ethacrynic acid (IV-1). For compound IV-3, we found that it inhibits jack bean urease by 50% at a concentration of 0.5 mM and even by 65% at a concentration of 1.0 mM (IC50 = 0.50 mM). Similar results were obtained for its chain length analogue IV-4 (48% inhibition at a concentration of 0.5 mM and 63% inhibition at a concentration of 1.0 mM). Even higher inhibitory activities could be found for compound IV-5 and its chain length analogue IV-6. These compounds are capable of inhibiting the enzyme by 64% or 65%, respectively, at the lower concentration of 0.5 mM and by 78% or 79%, respectively, at the higher concentration of 1.0 mM (IV-5, IC50 = 0.15 mM; IV-6, IC50 = 0.13 mM). Similar results were found for compounds IV-7 and its chain length analogue IV-8. Compound IV-7 inhibited jack bean urease by 62% (0.5 mM) and by 76% at a concentration of 1.0 mM (IC50 = 0.18 mM) and compound IV-8 showed 61% inhibition at a concentration of 0.5 mM and 75% inhibition at a concentration of 1.0 mM (IC50 = 0.21 mM). As a control, hydroxyurea (IC50 = 0.21 mM) and acetohydroxamic acid (IC50 = 0.05 mM), both known inhibitors of jack bean urease, were used. After an incubation time of 1 hour, the inhibitory activity of the most potent compounds (IV-5-8) was equal or better than that of hydroxyurea. However, they are three to four times less active than acetohydroxamic acid. An early conclusion is that the chain length of the acyl group does not seem to have a significant effect on the inhibitory activity of the corresponding compound, whereas the substitution pattern at the aromatic ring of the ethacrynic acid seems to have a great influence on the activity of the corresponding compound (chloro- versus hydrogen- versus methoxy substituent).
Inhibition of jack bean urease by ethacrynic acid (IV-1) and seven of its analogues (IV-2-8) at different incubation times.
| Compound | IC50 (mM) | |||
| 1 ha | 2 ha | 3 ha | 4 ha | |
| IV-1 (Ethacrynic acid) | 1.21 | 0.51 | 0.32 | 0.25 |
| IV-2 | 1.32 | 0.55 | 0.36 | 0.28 |
| IV-3 | 0.50 | 0.81 | 2.21 | − |
| IV-4 | 0.48 | 0.89 | 2.25 | − |
| IV-5 | 0.15 | 0.12 | 0.10 | 0.08 |
| IV-6 | 0.13 | 0.11 | 0.09 | 0.05 |
| IV-7 | 0.18 | 0.16 | 0.10 | 0.07 |
| IV-8 | 0.21 | 0.20 | 0.17 | 0.10 |
| Hydroxyurea | 0.21 | 0.20 | 0.21 | 0.22 |
| Acetohydroxamic acid | 0.05 | 0.04 | 0.04 | 0.05 |
a Preincubation time.
We were now interested if an increase in preincubation time has an effect on the inhibition of the enzyme. Therefore, we investigated the inhibitory activities of our compounds at various preincubation times (1, 2, 3, and 4 hours). Much to our delight, the inhibitory activities of ethacrynic acid (IV-1) and its chain length analogue (IV-2) were significantly increased at higher incubation times. The highest activity observed was after a preincubation of 4 hours. After this preincubation time, ethacrynic acid (IV-1) inhibited the enzyme by 71% at a concentration of 1.0 mM (IC50 = 0.25 mM). Surprisingly, with increasing preincubation time, the activities of the other compounds (IV-5-8) did not increase as significantly as for ethacrynic acid and its chain length analogue after an incubation time of 4 hours (IV-5, IC50 = 0.08 mM; IV-6, IC50 = 0.05 mM, IV-7, IC50 = 0.07 mM; IV-8, IC50 = 0.10 mM) (Table 1). The observation we made for compounds IV-3 and IV-4 was completely unexpected. The inhibitory activity of these compounds decreased from 48% inhibition (0.5 mM, 1 hour preincubation) to nearly no observable activity after a preincubation of 4 hours. We speculated that compounds IV-3 and IV-4 were degraded by the enzyme, which was affirmed by the fact that we were able to detect the presence of compounds IV-3 and IV-4 in the assay mixture after the preincubation of 1 hour, but not after 4 hours of incubation. The resulting product of the degradation couldn’t be identified. As a control, after a preincubation of 4 hours, we added more of compound IV-3 and IV-4 and could observe a returning inhibitory activity. Why this decomposition happens for compounds IV-3 and IV-4 and not for the chloro- or methoxy substituted compounds is not understood yet. It is noteworthy that all of the tested compounds inhibit the urease reversibly. Adding more urea to the assay mixture resulted in further activity of the enzyme.
In another experiment, we wanted to determine, if the addition of sulfhydryl compounds, such as benzyl mercaptan or cyclohexyl mercaptan, reduces the inhibitory activity of our compounds or even prevents the inhibition of jack bean urease. Sulfhydryl compounds are Michael donors and can attack the β-carbon of the α,β-unsaturated carbonyl unit, thereby preventing its reaction with cysteine residues in the active site of the jack bean urease The results from our enzyme assays show that one equivalent of the corresponding sulfhydryl compound (benzyl mercaptan or cyclohexyl mercaptan) affected the inhibitory activity of ethacrynic acid IV-1 and its analogues IV-3, IV-5, IV-7 significantly. We preincubated the enzyme with the respective compounds IV-1, IV-3, IV-5, and IV-7 at various concentrations in the presence of one equivalent of the corresponding sulfhydryl compound (benzyl mercaptan or cyclohexyl mercaptan, respectively) for 1 and for 4 hours and found that the inhibitory activity of the compounds was reduced by approximately half of their original activity (Table 2). As expected, the inhibitory activity of our control (acetohydroxamic acid) was not affected by the additives, no loss of activity could be observed. This is an indication that the α,β-unsaturated carbonyl unit is responsible for the inhibitory activity of our compounds described above. In order to verify our hypothesis, we tested all eight precursors (III-1-8), lacking the α,β-unsaturated carbonyl unit (Scheme 1) for their inhibitory activity on jack bean urease. As expected, none of the tested compounds were able to measurably inhibit the urease, not even at concentrations higher than 1.0 mM.
Effects of additives on the inhibitory activity of ethacrynic acid (IV-1) and three of its analogues (IV-3, IV-5, and IV-7) against jack bean urease at different incubation times.
| Compound | Additives | IC50 (mM) | |
| 1 ha | 4 ha | ||
| IV-1 (Ethacrynic acid) | None | 1.21 | 0.25 |
| Benzyl mercaptan (1 equiv) | 2.65 | 0.50 | |
| Cyclohexyl mercaptan (1 equiv) | 2.48 | 0.55 | |
| IV-3 | None | 0.50 | − |
| Benzyl mercaptan (1 equiv) | 1.15 | − | |
| Cyclohexyl mercaptan (1 equiv) | 1.11 | ||
| IV-5 | None | 0.15 | 0.08 |
| Benzyl mercaptane (1 equiv) | 0.35 | 0.20 | |
| Cyclohexyl mercaptan (1 equiv) | 0.28 | 1.17 | |
| IV-7 | None | 0.18 | 0.07 |
| Benzyl mercaptane (1 equiv) | 0.35 | 0.15 | |
| Cyclohexyl mercaptan (1 equiv) | 0.34 | 0.16 | |
| Acetohydroxamic acid | None | 0.05 | 0.01 |
| Benzylmercaptane (1 equiv) | 0.05 | 0.01 | |
| Cyclohexyl mercaptan (1 equiv) | 0.05 | 0.01 |
a Preincubation time.
4 Conclusion
In summary, we have tested ethacrynic acid (IV-1) and seven of its analogues (IV-2-8) for their potency to inhibit jack bean urease. We could demonstrate that the inhibitory activities of ethacrynic acid (IV-1) and its chain length analogue (IV-2) were significantly increased with higher incubation times. The highest activity observed was after a preincubation of 4 hours (IC50 = 0.25 mM or IC50 = 0.28 mM, respectively). However, the highest inhibitory activity was found for compound IV-6 (IC50 = 0.05 mM), compound IV-7 (IC50 = 0.07 mM), compound IV-5 (IC50 = 0.08 mM), and compound IV-8 (IC50 = 0.10 mM) after a preincubation time of 4 hours. It is noteworthy that all four compounds possess a methoxy group at the aromatic system. Compounds IV-3 (IC50 = 0.50 mM, 1 h preincubation) and IV-4 (IC50 = 0.48 mM, 1 h preincubation) are also capable of inhibiting jack bean urease. However, their inhibitory activity is reduced with increasing incubation time. It can be speculated that these compounds are degraded by the enzyme. Further investigations to account for these findings are necessary. Due to the distinct inhibitory activities of compounds IV-5, IV-6, IV-7, and IV-8, it can be speculated that the substituents attached to the aromatic system (chloro- versus hydrogen- versus methoxy substituent) have a great influence on the activity of the corresponding compound. A possible explanation for these distinct inhibitory activities is that methoxy groups favor the Michael addition reaction due to the fact that they activate the aromatic system through a resonance effect. On the other hand, the chloro substituents deactivate the ring through an electron-withdrawing inductive effect and thereby possibly disfavoring the Michael addition reaction. Further structure–activity relationship experiments have to be performed in order to get a better understanding of the influence of the substituents. The addition of sulfhydryl compounds reduces the activity of ethacrynic acid and its analogues significantly. This is insofar not surprising since the sulfur of the sulfhydryl compound can attack the β-carbon of the α,β-unsaturated carbonyl unit, thereby preventing its reaction with cysteine residues in the active site of the jack bean urease. Testing precursors of our compounds which lack the α,β-unsaturated carbonyl unit (III-1-8) for their inhibitory activity on urease reveals the importance of the α,β-unsaturated carbonyl unit because none of them show a measurable inhibition of the enzyme. Further investigations to design even more potent ethacrynic acid analogues for the inhibition of jack bean urease are currently underway in our laboratory.
Acknowledgements
The financial support of the US National Institutes of Health (P20 RR016480) under the INBRE program of the National Center for Research Resources (NCRR) is greatly appreciated.


