1 Introduction
Anvillea garcinii subsp. radiata (Cosson & Durieu) is a wild plant from the Asteraceae family that grows predominantly in the steppes of North Africa (Morocco and Algeria) and found in areas of the Middle East. Anvillea radiata is a small woody shrub, densely branched, 20–50 cm high. The leaves are green-gray, small, and roughly triangular, with a large petiole and strongly toothed limb. The big solitary capitules have a diameter of 3–5 cm, with long ligules. The flowers are all yellow-orange, the outside one 25 mm long. It usually flowers in spring, but can flower throughout the year. It is widely used in traditional medicine for the treatment of dysentery, gastric-intestinal disorders, and chest cold [1] and has been reported to have hypoglycemic activity [2] as well as antifungal activity [3,4]. A. radiata has been previously reported in the literature to contain mainly germacranolide compounds. From different chloroform extracts of the aerial parts, six germacranolides have been isolated. They have been identified as 9α-hydroxyparthenolide [5] 9β-hydroxyparthenolide [6], 9β-hydroxy-1β,10α-epoxyparthenolide [6], 9α-hydroxy-1β,10α-epoxyparthenolide [7], 8α,9α-epoxyparthenolide [8], parthenolid-9-one [7] and cis-parthenolid-9-one [9]. Germacranolides showed anti-inflammatory and antitumor activities and presented a great interest [10].
A. radiata aerial parts also contain phenolic compounds that are less studied than germacranolides. Only two papers reported the presence of aglycone or glycoside flavonoid compounds in A. radiata [11,12] and the identification of all this molecular family is incomplete. Phenolic compounds are well known for their beneficial effects on human health and their ability to limit damage from oxidative stress due to radical species [13]. As a consequence, the aim of this work was to characterize the phenolic composition of A. radiata aerial parts in order to gain better insight into the molecular content of this plant and contribute to a better phytochemical knowledge and use of this wild indigenous plant with medicinal activity.
Precedent works [11,12] described long and fastidious multi-step methodologies using maceration, liquid-liquid extraction, purification by liquid chromatography (LC) on open columns, preparative LC and Thin Layer Chromatography (TLC) to purify and isolate some individual molecules before their identification as flavonoids by 1H NMR. For some of them, less than 1 mg was lastly obtained making difficult their structural identification. Furthermore these methodologies were plant material, solvent and time consuming.
In the present work, we developed a simplified dereplication procedure to offer the possibility of obtaining structural information on all the phenolic constituents directly on-line. Selective extraction of flavonoids was carried out from A. radiata using accelerated solvent extraction (ASE) in two steps to remove, in a first step, major germacranolides present in the plant material and then to obtain from the plant residue free of germacranolides, an extract enriched in flavonoids. Rapid identification of compounds was then managed directly on the crude methanolic extract using HPLC-ESI/MS/MS and HPLC-High- Resolution MS (HPLC-HRMS) and without any need of further pre-treatment or prior compound isolation. This methodology was develop and applied to A. radiata aerial parts since the whole is frequently used in traditional medicine and would enter in cosmetic applications, then it was extend to each organ to qualitatively compare their phytochemical composition.
2 Materials and methods
2.1 Plant material
Aerial parts (flowers, leaves and stems) of A. radiata (Coss.&Durieu) were collected during the flowering period in May 2012 and May 2013, from Errachidia Road P21, Morocco. GPS coordinates were latitude (32.204086355917944) and longitude (−4.383201599121094). A voucher specimen has been deposited in the Herbarium of Scientific Institute, Rabat, Morocco.
The harvest parts were screened and free of contaminating portions then shade dried at room temperature. After drying, the plant material was stocked in the dark in the lab and was ground into fine powder using a basic grinder just before extraction.
2.2 Chemicals
Chloroform, methanol and formic acid were of analytical grade and provided by SDS Carlo Erba (Val-de-Reuil, France). Water was purified (resistance < 18 Ω) from ultrapure water using an Elgastat UHQ II system (Elga, Antony, France). The isorhamnetin, isorhamnetin-3-O-glucoside and chlorogenic acid were purchased from Extrasynthese (Genay, France).
2.3 Extraction procedure
An ASE 100 system from Dionex (Voisins le Bretonneux, France) with 34 mL stainless steel ASE vessels was used for the pressurized extraction. 2.5 g of powder material was mixed with Na2SO4 (2.5 g) as a dispersant agent and extracted successively with two different solvents (chloroform and then methanol). The following standard parameters recorded in ASE were applied: extraction time 5 min, flush volume of 65% and purge with nitrogen gas during 100 s at the end of each extraction. Extractions were carried out at 40 °C to avoid potential compound degradation with temperature and under a pressure of 100 bar. For each solvent two static cycles were performed. The liquid extracts (chloroform and methanol extracts) were then evaporated using a rotary evaporator (Buchi Labortechnik AG, Switzerland) under vacuum to obtain dried crude extracts before calculating an extraction yield.
2.4 Acid hydrolysis
Total acid hydrolysis of the methanol extract of aerial parts was carried out under the same conditions as reported in Ref. [14]. 1 mL of 4 M HCl was added to 1 mL of 10 g/L methanol extract solution. This mixture solution was kept in a closed vial for 30 min at 85 °C and directly analyzed by LC-DAD-ESI-MS/MS.
2.5 HPLC analysis
The HPLC system consisted of an Agilent Technologies Series 1100 system (Palto Alto, CA, USA) equipped with a binary pump. Chromatographic separations were performed on a Purospher® RP18e column (125 × 4 mm, 5 μm) (VWR Fontenay sous bois, France), at room temperature. The mobile phase was delivered at a flow rate of 1 mL min−1 and consisted of ultrapure water (solvent A) and methanol (solvent B) both acidified with 0.1% formic acid using gradient elution (0–40 min: 5–90% B, 40–50 min: 90% B). Equilibration time was of 10 min between two successive injections. The sample injection volume was 20 μL and the extract concentration was 1 mg/mL dissolved in the mobile phase. The detection was done with a UV-Visible Spectroflow 783 (Bristol, CT, USA) from Kontron Instruments (Montigny Le Bretonneux, France) set at 366 nm to detect flavonoid compounds and with an evaporative light scattering detector (ELSD) type SEDEX 55 from SEDERE (Alfortville, France) used under the following conditions: nebulization temperature 52 °C; nebulization gas pressure 2.2 bar; and gain 10 allowing the detection of germacranolides that do not possess any chromophore group.
2.6 Identification and structural characterization
2.6.1 HPLC-DAD-ESI-MS/MS
HPLC-DAD-ESI/MS analyses were done using an Agilent Technologies 1100 Series system (Palto Alto, CA, USA) equipped with a binary pump, an automatic injector (Vinj = 20 μL) and an interface for DAD and MS detectors. The same chromatographic conditions as described above were applied and the mass spectra were obtained in the negative ionization mode on an API 3000 triple quadrupole mass spectrometer (AB SCIEX, Foster City, CA, USA) equipped with a turbo ion spray source and analysis software version 1.4.2 (Applied Biosystem MDS Sciex). The 1 mL/min flow rate from the HPLC device was split to a flow rate of approximately 0.3 mL/min directed to the MS system. In the analysis of a single scan (Q1), the quadrupole was operated under the following conditions: curtain gas, nebulizer gas flow rate of 1.2 L/min; ion spray voltage –4.2 kV, source temperature 300 °C, declustering potential –100 V, focusing potential – 400 V and entrance potential –10 V. The full scan mass spectra were obtained in a scan range from 100 to 1000 m/z with a step size of 0.5 and a scan cycle time of 0.605 s. The MS/MS scan mass spectra were recorded with nitrogen as collision gas and a collision energy of −30 eV from 100 to 550 m/z.
2.6.2 High resolution mass spectrometry (HRMS) analysis
HRMS analyses were performed on the methanol extract using a maXis UHR-Q-TOF mass spectrometer (Bruker, Bremen, Germany). The mass spectrometer was operated in the negative electrospray ionization mode and acquired data at 1 Hz in a mass range from 50 to 2500 m/z. The capillary voltage was set at –4.5 kV, the flows of nebulizing and drying gas (nitrogen) were respectively set at 1.2 bar and 8.5 L/min, and the capillary temperature of the ESI source was 200 °C. The accurate mass data of the molecular ions were processed through the Data Analysis 4.0 software (Bruker Daltonik), which provided a list of possible elemental formulae using the SmartFormula Editor tool.
3 Results and discussion
3.1 Selective extraction of phenolic compounds
According to the literature A. radiata contains germacranolides present in high amount and less abundant flavonoids. Germacranolides are interesting molecules with numerous biological activities and have been largely studied in A. radiata. Moreover their high content makes difficult the access to minor flavonoid compounds. Thus deeper analysis of flavonoids could be more easily managed if the germacranolides were previously removed from the material and the studied extract was enriched in phenolic compounds. Germacranolides are frequently extracted with chloroform whereas flavonoids are preferentially extracted with methanol or ethanol [7,8,11]. Consequently a selective extraction of flavonoids was first attempted extracting A. radiata aerial parts with a polar solvent such as methanol and water/methanol mixtures [15]. Unfortunately under these conditions, both kinds of compound families were simultaneously extracted even if germacranolides seemed to be less polar than flavonoids. Liquid-liquid partition performed on this first methanolic extract and using different less polar solvents such as hexane, CHCl3, ethyl acetate or butanol did not lead to a selective separation of the two families. However during partition germacranolides showed a good affinity for chloroform in accordance with their extraction solvent in the literature [7,8]. Thus a two-step extraction of A. radiata aerial parts was investigated using first an extraction with chloroform to remove mainly germacranolide compounds and then a second extraction with methanol in order to recover flavonoids from the plant residue.
Accelerated solvent extraction (ASE) appeared to be the most suitable technique to perform this multi-step extraction protocol. Indeed the ASE cell was filled with raw material and extractions with different solvents could be performed by just changing solvent bottles without any vegetal treatment or filtration. Thereby chloroform extraction was first performed on A. radiata aerial parts and then methanol extraction was managed, directly on the residue, under the same conditions. After two extraction cycles the plant material was exhausted. Two different limpid liquid extracts (CHCl3 and MeOH) were recovered independently in separated bottle collection. The extraction yield of A. radiata aerial parts, calculated as the ratio of the dried extract weight to the dried plant material weight, was different according to the solvent used. It was about 18.6% (w/w) with chloroform and 8.3% (w/w) with methanol.
Both extracts were analyzed using HPLC-UV-ELSD. ELSD is convenient to detect germacranolides that do not possess any chromophore group while UV is a more sensitive detector for flavonoids that selectively absorb at 366 nm. Fig. 1A depicts the HPLC-ELSD chromatographic profile of the chloroform ASE-extract. Two kinds of compounds were observed in the chloroform ASE extract. The first one corresponds to intense peaks A and B (Fig 1A) only detected with ELSD whereas the second one is constituted of solutes eluted in two additional peaks at 26–27 min and better detected with UV set at 366 nm (not shown). Their very low concentration in the chloroform ASE-extract leads to their poor detection with ELSD. According to previous work [16], it can be assumed that peaks A and B correspond to the two major germacranolides (9α-and 9β-hydroxyparthenolide) present in A. radiata aerial parts while the two additional peaks detected at 26–27 min were associated to elution of some flavonoids extracted in a very weak proportion.
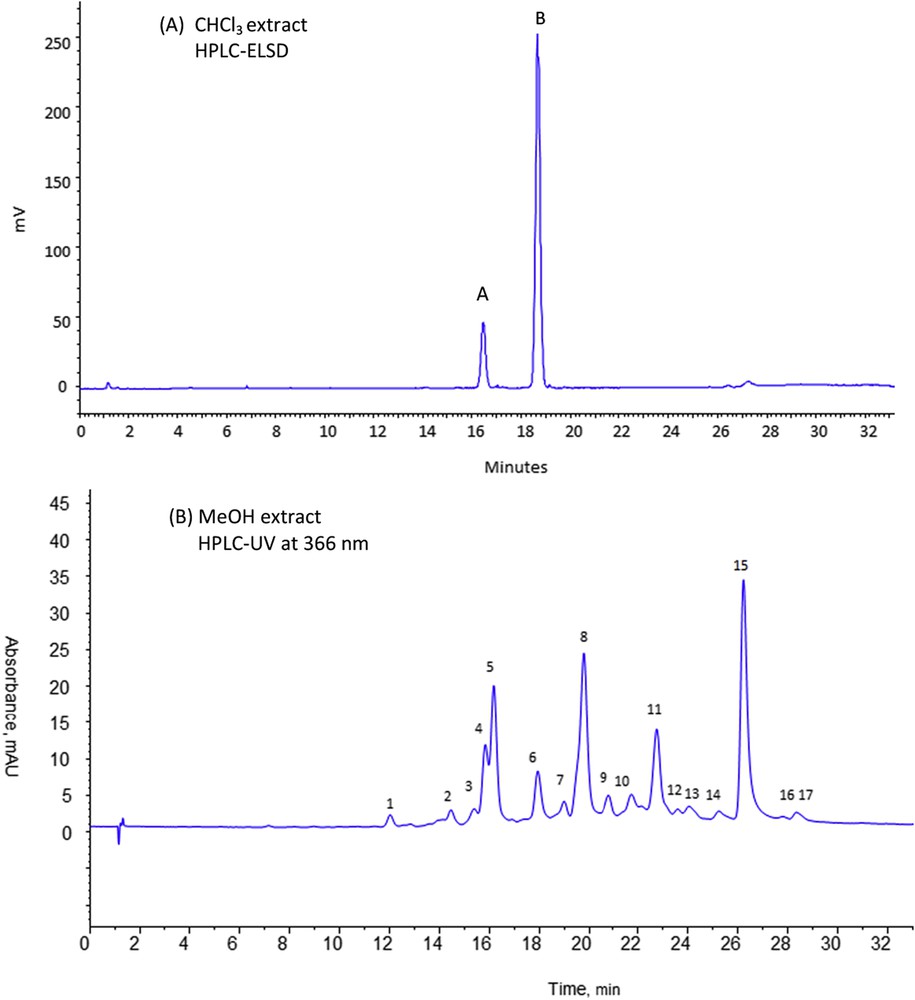
HPLC-DAD-ELSD analyses of chloroform (A) and methanol (B) extracts of Anvillea radiata aerial parts. Column purosphere RP18e (125 × 4 mm, 5 μm). Mobile phase A: 0.1% formic acid in water, B: 0.1% formic acid in methanol, gradient elution: 0–40 min, 5–90% B, 40–50 min, 90% B, equilibration time 10 min. Flow rate: 1 mL min−1, room temperature, Vinj = 20 μL. Extract concentration 1 mg/mL in mobile phase; (A) ELSD detection 52 °C, 2.2 bar, gain 10; (B) UV detection at 366 nm.
Fig. 1B reports the HPLC-UV chromatographic profiles of the methanol ASE-extract with UV set at 366 nm. The methanol ASE-extract is richer in compounds well detected by UV at 366 nm. Furthermore, ELSD detection confirms the efficiency of the first extraction step to remove all the major germacranolides as no ELSD signal corresponding to these molecules was observed during the analysis of the methanolic ASE-extract. From the chromatographic fingerprint depicted in Fig 1B, 17 chromatographic peaks were separated, showing the richness of the methanolic extract. In view of the characteristic absorbance spectra obtained for these solutes, the presence of flavonoids could be presumed.
Finally, the developed ASE methodology leads to two types of extracts: the first one containing the valuable germacranolides and the second one selectively enriched in flavonoids that could be further studied.
The developed procedure was also applied to extract separately each organ (flowers, leaves and stems) of A. radiata. After HPLC-UV-ELSD analysis of each CHCl3 and MeOH extracts, similar conclusions could be observed in comparison with the analysis of whole aerial parts. The three CHCl3 extracts contained the same two major germacranolides while the three MeOH ones contained flavonoids indicating that both kinds of compounds were present in all the aerial plant parts. All the three different methanolic organ fingerprint profiles (Fig. 2) show chromatographic peaks eluted over the same range of polarity. Same compounds seem to be present in the different organs however in variable proportions depending on the organ. Chromatographic profiles of stems and leaves are very close with the same intense peaks (4, 5, 6). The flower profile seems to be quite different with a main peak (8).
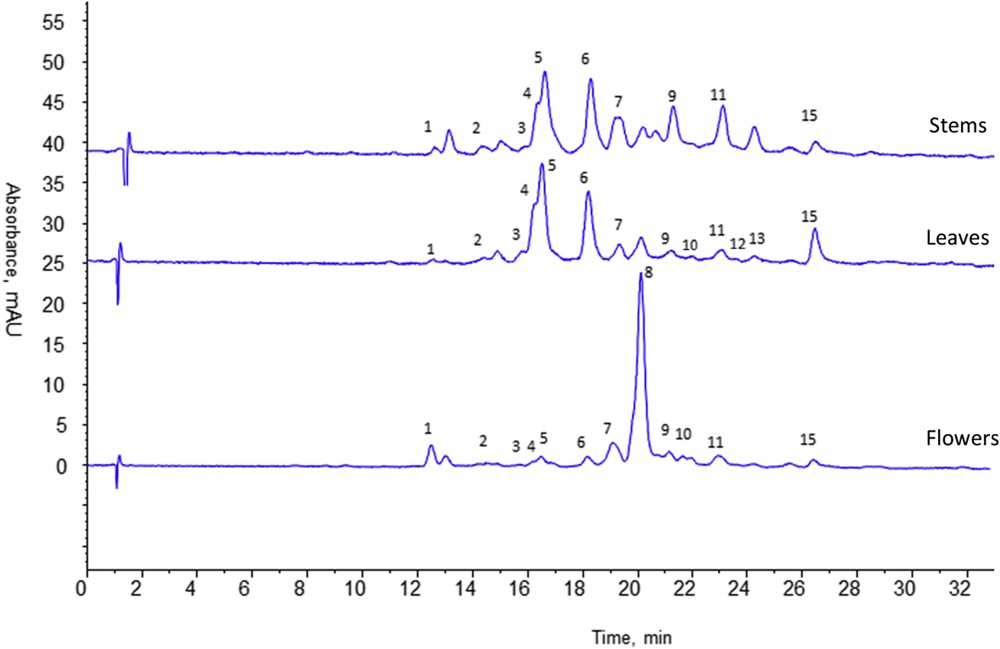
HPLC analyses of methanol extracts of flowers, leaves and stems of Anvillea radiata. Column purosphere RP18e (125 × 4 mm, 5 μm). Mobile phase A: 0.1% formic acid in water, B: 0.1% formic acid in methanol, gradient elution: 0–40 min, 5–90% B, 40–50 min, 90% B, equilibration time 10 min. Flow rate: 1 mL/min, room temperature, Vinj = 20 μL. Extract concentration 1 mg/mL in mobile phase; UV detection at 366 nm.
3.2 Identification and structural characterization
In order to identify flavonoid compounds HPLC-DAD-ESI/MS/MS and HPLC-HRMS analyses on the aerial part methanol extract were managed in a full scan mode to obtain the mass spectra for each chromatographic peak. A comparison of the two ESI ionization modes confirmed that the ESI analysis in the negative ionization mode provided the most exhaustive fingerprint regarding the number and intensity of phenolic peaks detected. Frequently full scan mass spectra presented the deprotonated molecule ion [M−H]‒ and in-source fragment ions. With the MS/MS analyses (parent and daughter scan analyses) the fragmentation pattern of each compound could be confirmed improving compound identification. Moreover, HPLC-HRMS analysis allowed obtaining the accurate mass and molecular formulae propositions from the SmartFormula tool of the data analysis software. Gathering information given by HPLC-HRMS and HPLC-DAD-ESI/MS/MS a confident identification of compounds could be proposed.
Untargeted analysis in HPLC-DAD-ESI/MS in the full scan mode showed the presence of two families of compounds. The first one was characterized by a UV spectrum with λ max around 280 and 320 nm and by a mass spectrum with an intense ion at m/z 191. These compounds presented similar behavior to chlorogenic acid. The second one showed a UV spectrum with a different shape with λ max around 350–360 nm and different ions in MS spectra; according to the literature these compounds could be supposed to be flavonoids [17].
3.2.1 Phenolic acid derivatives
The hypothesis of the chlorogenic acid content could be confirmed by the injection of chlorogenic acid (5-O-caffeoylquinic acid) standard that is eluted at 12.05 min. UV and mass spectra recorded for chlorogenic acid were in accordance with those observed for the first group of compounds in the methanol extract. λ max were 300 and 330 nm and the MS spectrum showed a deprotonated molecule ion [M−H]‒ at m/z 353 and an intense fragment ion at m/z 191 due to the loss of the caffeoyl group, the negative charge remaining on the quinic acid.
Seven compounds (1, 3, 7, 8, 9a, 10b and 12) presented in Table 1 showed similar UV spectra and fragmentation patterns and were all identified as chlorogenic acid derivatives.
HPLC-DAD-ESI/MS/MS and HPLC-HRMS analyses of phenolic acids and flavonoids from the Anvillea radiata aerial part methanol extract, molecular formula proposed by the MS software and possible identification of main compounds.
| Peak no. | Compounds | tr (min) | ƛ max (nm) | [M−H]− (m/z) | Fragments ions (m/z) | Accurate mass [M−H]− | Proposed molecular formula | Identification | Ref |
| 1 | 1 | 12.05 | 220–290–330 | 353 | 191 | 353.0878 | C16H18O9 | 5-O-Caffeoylquinic acida | [18] |
| 2 | 2 | 14.48 | 230–270–340 | 655 | 493, 330 | 655.1516 | C28H32O18 | Patuletin diglucoside | [11] |
| 3 | 3 | 15.41 | 225–270–330 | 367 | 191 | 367.1035 | C17H20O9 | Feruloylquinic acid | [19] |
| 4 | 4 | 15.86 | 230–260–360 | 639 | 477, 315, 300, 273, 151 | 639.1567 | C28H32O17 | Isorhamnetin-3-diglucoside | [11] |
| 5 | 5 | 16.20 | 224–255–350 | 669 | 507, 344 | 669.1672 | C29H34O18 | Spinacetin-3-diglucoside | [11] |
| 6 | 6 | 17.96 | 230–270–350 | 711 | 549, 507, 386, 344 | 711.1791 | C31H36O19 | Spinacetin acetyl-diglucoside | [24,25] |
| 7 | 7 | 19.01 | 230–280–330 | 515 | 353, 191 | 515.1195 | C25H24O12 | Di-O-caffeoylquinic acid | [18,19] |
| 8 | 8 | 19.80 | 220–290–330 | 515 | 353, 191 | 515.1195 | C25H24O12 | Di-O-caffeoylquinic acid | [18,19] |
| 9 | 9a | 20.82 | 230–290–340 | 515 | 353, 191 | 515.1195 | C25H24O12 | Di-O-caffeoylquinic acid | [18,19] |
| 9b | 493 | 331 | 493.0988 | C22H38O12 | Patuletin glucoside | ||||
| 9c | 463 | 301 | 463.0882 | C21H20O12 | Quercetine-3-glucoside | [11] | |||
| 9d | 477 | 315, 300 | 477.1038 | C22H22O12 | Isorhamnetin or nepetin hexoside | ||||
| 10 | 10a | 21.74 | 230–290–330 | 477 | 315, 300 | 477.1038 | C22H22O12 | Isorhamnetin or nepetin hexoside | [12,25] |
| 10b | 529 | 367, 191 | 529.1351 | C26H26O12 | Feruloyl-caffeoylquinic acid | [19] | |||
| 11 | 11a | 22.75 | 255–268–350 | 507 | 345 | 507.1144 | C23H24O13 | Spinacetin-7-glucoside | [11] |
| 11b | 477 | 315, 300 | 477.1038 | C22H22O12 | Isorhamnetin 3-glucosideb | [12,25] | |||
| 12 | 12 | 23.59 | 230–290–330 | 543 | 367, 191 | 543.1508 | C27H28O12 | Diferuloylquinic acid | [20] |
| 13 | 13 | 24.04 | 230–280–330 | 477 | 315, 300 | 477.1038 | C22H22O12 | Isorhamnetin or nepetin glucoside | [25] |
| 14 | 14 | 25.24 | 230–280–330 | 331 | 316 | 331.0459 | C16H12O8 | Patuletin | [11] |
| 15 | 15 | 26.23 | 220–270–350 | 315 | 301 | 315.0510 | C16H12O7 | Nepetin | [11] |
| 16 | 16 | 28.20 | 220–250–370 | 315 | 301, 273, 151 | 315.0510 | C16H12O7 | Isorhamnetinc | |
| 17 | 17a | 28.35 | 228–290–320 | 329 | 314, 299 | 329.0667 | C17H14O7 | Jaceosidin | [11,22] |
| 17b | 345 | 330, 315 | 345.1344 | C17H14O8 | Spinacetin | [11] |
a Chlorogenic acid.
b Isorhamnetin 3-glucoside.
c Isorhamnetin.
Compound 1 could be identified as 5-O-caffeoylquinic (or chlorogenic) acid, C16H17O9. It was eluted at the same retention time and presented the same mass spectrum as the corresponding chlorogenic acid standard [18].
Compounds 7, 8 and 9a all exhibited the same MS spectra with a [M−H]‒¯ ion at m/z 515.1195 (C25H23O12) and two fragment ions at m/z 353 and 191, suggesting the loss of two caffeoyl residues. These compounds were identified as di-O-caffeoylquinic acid isomers [18,19].
Compounds 3 and 12 showed a [M−H]‒ ion at m/z 367.1035 (C17H19O9) and at 543.1508 (C27H27O12) respectively. Mass differences between the [M−H]‒ ion and the corresponding fragment ions for each compound indicated the loss of the feruloyl group (176 Da). Thus they were identified as feruloylquinic and diferuloylquinic acids respectively [19,20].
Compound 10b, identified as feruloyl-caffeoylquinic acid, presented a [M−H]‒ ion at m/z 529.1351 (C26H25O12) and two fragment ions at m/z 367 and 191 suggesting the loss of one caffeoyl and one feruloyl residue respectively [19].
Identification of these compounds is fully consistent with the chromatographic retention due to their relative polarity: the compound retention increases with the number of carbon from the caffeoyl to the diferuloyl substituent.
3.2.2 Flavonoids
Identification of flavonoid compounds was less evident since mass spectra presented more fragment ions suggesting both the loss of sugar moieties and that of methyl groups. Moreover some compounds seemed to be coeluted leading to overlapping of different fragmentation patterns on the same mass spectrum. Thus in order to determine the number and the nature of the different flavonoids contained in A. radiata, an acid hydrolysis of the crude methanol extract was first carried out. By this way, the sugar moieties linked to the flavonoid genin were removed and it was possible to determine the flavonoids present in the extract under their simplified aglycone forms. The hydrolyzed extract was analyzed using HPLC-DAD-ESI/MS and the obtained UV chromatogram at 366 nm is presented in Fig. 3. Compared to the crude extract analysis, the hydrolyzed extract chromatogram at 366 nm showed four major peaks with higher intensity and some other minor peaks corresponding to the derivatives of chlorogenic acid and residues of glycosylated flavonoids unhydrolyzed. The four major compounds had the same retention time and the same mass spectrum as those of the last eluted compounds in the crude methanol extract (peaks 14, 15, 16 and 17 in Fig 1 and Table 1) highlighting the presence of aglycone flavonoids in the crude extract.
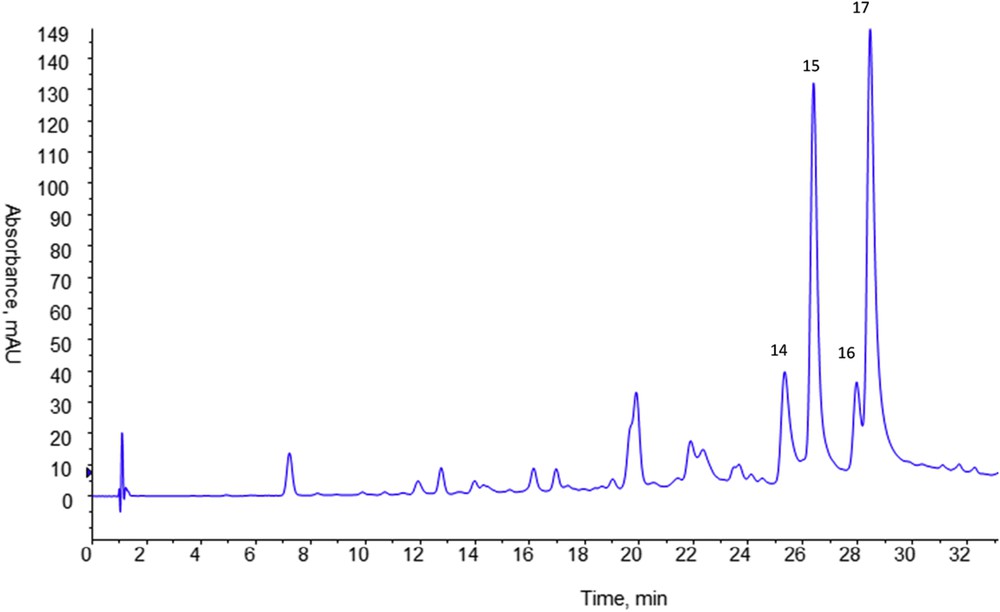
HPLC analysis of the methanol extract of Anvillea radiata aerial parts after acid hydrolysis. Column purosphere RP18e (125 × 4 mm, 5 μm). Mobile phase A: 0.1% formic acid in water, B: 0.1% formic acid in methanol, gradient elution: 0–40 min, 5–90% B, 40–50 min, 90% B, equilibration time 10 min. Flow rate: 1 mL min−1, room temperature, Vinj = 20 μL. UV detection at 366 nm.
Compound 14 presented a deprotonated molecule ion [M–H]‒ at m/z 331.0459 (C16H11O8) and fragment ions at m/z 316 indicating the loss of one methyl group. This fragmentation pattern was similar to that of patuletin already reported in A. radiata [11].
Compounds 15 and 16 showed the same deprotonated molecule ion [M−H]‒ at m/z 315.0510 corresponding to the same molecular formula C16H11O7 and the same main in-source fragment at m/z 301 [M–H–CH3]–¯. Nepetin and isorhamnetin could correspond to this formula. If nepetin has been previously described in A. radiata [11], the presence of isorhamnetin has been reported only under its glycosylated form. HPLC-DAD-ESI/MS analysis of the isorhamnetin standard molecule allowed after comparison of the retention time, UV and mass spectra, and identification of compound 16 as isorhamnetin. Then, it could be supposed that compound 15 corresponds to nepetin [11]. This identification was also supported by the presence of low intensity fragment ions at m/z 273 [M–H–CO]–¯ and 151 [1,3A]–¯ in the mass spectrum of isorhamnetin [21].
Peak 17 was associated to two different compounds. Compounds 17a and 17b gave a [M−H]‒ ion at m/z 329.0667 (C17H13O7) and 345.1344 (C17H13O8) respectively. The main fragment ion corresponding to each base peak was attributable to the loss of two methyl groups. The compounds 17a and 17b were identified respectively as jaceosidin and spinacetin, already described in A. radiata [11,22].
The structures of these four aglycone flavonoids are given in Fig. 4. It could be now supposed that other flavonoid derivatives observed in the crude methanol extract should correspond to glycoside derivatives of these four aglycone molecules.
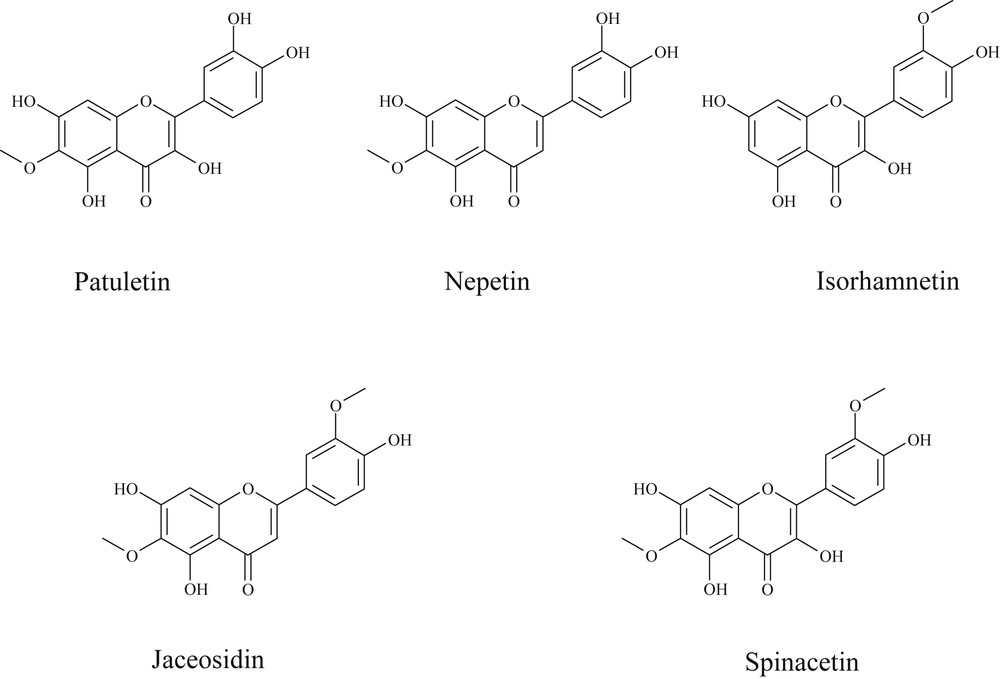
Structures of aglycone flavonoids identified in the Anvillea radiata aerial part methanol extract.
Analysis of the crude methanol extract led to identification of 11 glycoside flavonoids. For each of them a [M−H]‒ ion was detected, and loss of sugar moieties induced the formation of [Y0-H]‒ or [Y0]‒ ions corresponding to the flavonoid genin. For compounds 2, 3a, 4 and 5 an intermediate fragment ion [Y1]‒ was also observed indicating that these compounds were diglycoside molecules [23]. The mass difference between these ions was 162 Da corresponding to hexose moieties. Following this fragmentation pattern compound 2 was identified as patuletin-3 or 7-diglucoside, compound 4 showed fragment ions corresponding to an isorhamnetin derivative and was identified as isorhamnetin-3-diglucoside, and compound 5 could be identified as spinacetin-3-diglucoside. These molecules have already been identified in A. radiata [11].
Thus first eluted flavonoid compounds correspond to diglycosylated derivatives. The elution order depends on the aglycone form polarity and follows the same order as the aglycone compounds in the hydrolyzed extract (patuletin first, then isorhamnetin and lastly spinacetin).
Compound 6 showed a [M-H]‒ ion at m/z 711.1791 (C31H35O19) and fragment ions at m/z 549 and 386 due to the loss of one or two hexoside moieties (162 Da). Additional fragment ions at m/z 507 and 345 due to a loss of 42 Da from the [M–gly–H]‒ and [M–2gly–H]‒ ions suggested the presence of an acetyl residue (COCH2) on the aglycon form that should be a spinacetin regarding the last ion [Y0]‒ at 345. Therefore, compound 6 was proposed as spinacetin-acetyl-diglucoside [24,25].
In Peak 9, three independent flavonoids 9b, 9c and 9d were coeluted. The first fragmentation pattern lead to identification of patuletin glucoside with [M−H]‒ at m/z 493.0988 (C22H37O12), [Y0]‒ at m/z 331 and [Y0-Me]‒ at m/z 316. The second compound with a [M−H]‒ ion at m/z 463.0882 (C21H19O12) and [Y0]‒ m/z 301 was identified as quercetine-3-glucoside, already described in A. radiata [11]. The last one corresponds to isorhamnetin or nepetin glucoside with a [M−H]‒ ion at m/z 477.1038 (C22H21O12), [Y0]‒ at m/z 315 and [Y0-Me]‒ at m/z 300. Due to the coelution of quercetine glucoside that showed similar fragment ions as isorhamnetin and the low intensity of lower mass fragment ions, it was not possible to distinguish between isorhamnetin and nepetin derivative.
Compounds 10a, 11b and 13 gave a [M−H]‒ ion at m/z 477.1038 (C22H21O12), [Y0]‒ at m/z 315 and [Y0-Me]‒ at m/z 300. All these compounds could correspond to isorhamnetin or nepetin hexoside derivatives. The standard molecule of isorhamnetin-3-O-glucoside, already described as presented in A. radiata, was injected in the chromatographic system and showed the same retention time as compound 11b, allowing a more consistent identification of this analyte. This molecule was coeluted with 11a proposed as spinacetin-7-glucoside [11].
According to these results, it was possible to confirm the presence of patuletin-3 or 7-diglucoside, isorhamnetin-3-diglucoside, spinacetin-3-diglucoside, patuletin-7-glucoside, quercetine-3-glucoside, spinacetin-7-glucoside, isorhamnetin-3-glucoside, nepetin and jaceosidin already described in A. radiata [11]. Moreover additional glycosylated or aglycone flavonoids have been identified thanks to the simplified dereplication procedure implemented in our work. Several chlorogenic acid derivatives were recovered for the first time in A. radiata and appeared among the major compounds of the methanol ASE-extract. In a previous paper [11], identification of flavonoid compounds in A. radiata was investigated after a fastidious procedure including a first methanol extraction of the dried plant material, then a liquid–liquid extraction (H2O–butanol) carried out on the methanol dried extract, and a purification of the butanol fraction on an open column. Under these conditions, it was possible that chlorogenic acids, higher polar solutes, were remained in the aqueous phase and then not detected in the butanol fraction. In our method, the direct analysis of the crude extract without any pre-treatment allowed a complete determination of all extracted phenolic compounds.
In order to evaluate the compound repartition in the different aerial plant parts, the organs were separated during the collect. Hence flowers, leaves and stems were collected, extracted and analyzed separately with the same HPLC-DAD-MS/MS methodology. Main compounds could be found in all part extracts. Table 2 shows compounds detected in aerial parts and their recovery in the three different organs. Among the aglycone flavonoids identified in the whole aerial parts, nepetin was the only one detected in all the organs. It was possible that the content of the other minor aglycone flavonoids was too low in each organ separately to be detected. Stems and leaves show similar chromatographic profiles. Isorhamnetin and spinacetin diglucoside derivatives seem to be the main compounds in these organs. The flower extract is more different with a di-caffeoylquinic acid as the main compound. Overall, this highlights the low phytochemical specificity of each organ and justifies the traditional use of A. radiata aerial parts as a whole rather than the use of one of its specific organ.
HPLC-DAD-ESI/MS/MS analyses of methanol extracts of the Anvillea radiata different organs. Identification in each organ of compounds detected in aerial parts.
| Peak | Compounds | Identification | Flowers | Leaves | Stems |
| 1 | 1 | 5-O-Caffeoylquinic acida | × | × | × |
| 2 | 2 | Patuletin diglucoside | × | × | × |
| 3 | 3 | Feruloylquinic acid | × | × | × |
| 4 | 4 | Isorhamnetin-3-diglucoside | × | × | |
| 5 | 5 | Spinacetin-3-diglucoside | × | × | × |
| 6 | 6 | Spinacetin acetyl-diglucoside | × | × | × |
| 7 | 7 | Di-O-caffeoylquinic acid | × | × | × |
| 8 | 8 | Di-O-caffeoylquinic acid | × | ||
| 9 | 9a | Di-O-caffeoylquinic acid | × | ||
| 9b | Patuletin-glucoside | × | × | × | |
| 9c | Quercetine-3-glucoside | × | × | × | |
| 9d | Isorhamnetin or nepetin glucoside | × | × | ||
| 10 | 10a | Isorhamnetin or nepetin glucoside | × | × | × |
| 10b | Feruloyl-caffeoylquinic acid | × | × | × | |
| 11 | 11a | Spinacetin-7-glucoside | × | × | × |
| 11b | Isorhamnetin-3-glucosideb | × | × | × | |
| 12 | 12 | Diferuloylquinic acid | × | ||
| 13 | 13 | Isorhamnetin or nepetin glucoside | × | ||
| 14 | 14 | Patuletin | |||
| 15 | 15 | Nepetin | × | × | |
| 16 | 16 | Isorhamnetinc | |||
| 17 | 17a | Jaceosidin | |||
| 17b | Spinacetin |
4 Conclusion
The development of a selective Accelerated solvent extraction (ASE) in two successive steps from the same plant sample associated to HPLC-DAD-MS/MS analysis of the obtained methanolic ASE extract has led to a deeper characterization of the phenolic content of A. radiata aerial parts. A tentative identification of 23 compounds including 7 phenolic acids and 16 flavonoids can be proposed. The identified phenolic acids corresponded to chlorogenic acid derivatives described for the first time in A. radiata. Flavonoid compounds were mainly di- and mono-glycosylated derivatives of patuletin, isorhamnetin, nepetin and spinacetin found also under their aglycon form. Some of them have been previously identified in A. radiata but the higher sensitivity of the simplified dereplication procedure and the direct crude extract analysis allowed the detection of more numerous and less abundant compounds. Analysis of separated plant organs (flowers, leaves and stems) showed that all the compounds seemed to be present in all plant parts and only differences in the relative abundance of molecules were observed. Leaves and stems appeared to be richer in di-glucoside derivatives of isorhamnetin and spinacetin while flower were richer in di-caffeoylquinic acid.
An HPLC analysis associating the use of two complementary mass spectrometers (tandem mass spectrometry and high resolution mass spectrometry) has shown to be a very useful tool for a confident identification of compounds in complex extracts. Furthermore, the better knowledge of the phenolic profile of A. radiata might contribute to the valorization of the bioactivity potential of this plant in cosmetic applications.
Acknowledgements
This work forms part of a scientific project (ValPAMMeT) concerning the valorization of aromatic and medicinal plants from the Meknès-Tafilalet region (Morocco). It was financially supported by the Conseil Régional du Centre-Val de Loire (France).


