1. Introduction
Liquid–liquid extraction is a very efficient technique for the partitioning and the separation of various compounds, especially for metal ions [1]. In particular, this technique has been investigated for the treatment of nickel [2], zinc [3], cupper [4] and lanthanide [5] -rich ores, among others. It is also a technique of choice for the recovery of valuable metals from several secondary sources [6, 7, 8, 9, 10, 11] and for nuclear fuel reprocessing [12, 13, 14]. Based on its success, this technique is the subject of an intense and vivid academic interest, both on the fundamental side, in view of the understanding of extraction mechanism [15, 16, 17], or on an applied perspective (see for example the patent CA2785001A1 published in 2018, for purifying uranium from nitric acid dissolution1 ). Through the years, very efficient systems, based on molecular organic solvents, have been designed for a wide variety of metal-containing aqueous phases to be treated. However, as ecological concerns have risen, molecular organic solvents such as dichloroethane, benzene and others are now recognized as toxic and dangerous compounds. Therefore, one of the biggest challenges now is to conceive liquid–liquid extraction systems (LLES) as efficient as before but complying as much as possible with the green chemistry concepts. In this respect, two other ways of performing liquid–liquid extraction are receiving increasing attention.
First, aqueous biphasic systems (ABS) have been already envisioned for metal extraction more than twenty years ago [18, 19, 20, 21] and interest in these systems is still very high [22, 23, 24, 25]. In fact, ABS have been known for long as the first proof of concept for the extraction of biological molecules can be traced back in the 50’s [26] but since the breakthrough of acidic-ABS which allow extraction of metals suffering hydrolysis [27], their use for metal extraction has gained even more interest [28]. Second, the so-called deep eutectic solvents (DES) are under the light, with hydrophobic DES mixtures able to extract metal ions [29, 30, 31, 32, 33]. It seems therefore that three clearly different categories of LLES, based on organic molecular solvents, ABS and DES can allow metal ion extraction from aqueous phases. In this respect, the first section of this paper will set the definitions to be used all throughout this paper and summarize the main accepted characteristics of these systems.
The first objective of this article is to convince the reader that these apparently well-defined categories are in fact blurred. Their most common definitions are unclear and lack precise quantitative descriptions, while the boundaries between them are very porous. Second, as these three categories could well be combined into a single one without any lack of information, it will be shown how it is possible to take advantage of the characterization method of one of these former categories for the understanding of all these systems and their unification.
2. Experimentals
Ultra-pure water (Millipore system, 18 M𝛺) has been used for all samples. Tetrabutylammonium chloride, N4444Cl, (Aldrich, purity ⩾ 97%) and decanoic acid, (Sigma-Aldrich, purity > 98%), noted as HDec in the following, have been used as received. A Fisherbrand balance (Analytical series, precision 0.0001 g) has been used to prepare the samples. The method to obtain the phase diagram of the ternary mixture H2O/HDec/N4444Cl partly takes advantage of a protocol already published for the mixing of N4444Cl and HDec [34]. First, several proportions of the two compounds N4444Cl and HDec have been poured in glass vials (from ca. 9 wt% to 87 wt% of HDec, total mass ca. 5 g, no water added) and heated above ca. 45 °C, to insure a complete melting. The samples were subjected to vigorous shaking for a few seconds just before being transferred into a thermostatic bath (T = 25 °C, precision ± 0.5 °C) and left to thermalize for at least half an hour after which a visual inspection allowed the determination of the sample state under three possibilities: (i) liquid monophasic (ii) liquid biphasic (iii) solid. Once the state of the samples was determined, water was added in the vials (from 0.5 to 1.2 g H2O per addition), and samples were again heated above 45 °C to insure melting of the N4444Cl–HDec components before being put in the thermostatic bath. The water addition procedure is repeated as many times as deemed necessary to delimit the zone of interest of the ternary system H2O/HDec/N4444Cl. This method differs from the traditional turbidity method used for ABS binodal determinations [35] and also differs from the battleship method recently proposed [36]. In this work, all the data are plotted using orthogonal 2D diagrams, in units of weight percent (wt%) and the corresponding triangular displays can be found in the supplementary material (Figures S1–S3). According to the procedure used in this work, in the phase diagram, the positions of the samples obtained by successive water additions are aligned and converge, at infinite water addition, towards pure water. Considering the plot obtained, the poetic name proposed for this method is the butterfly wing protocol (see Figure S4). As compared to the battleship method, the advantages of the butterfly wing protocol are a more systematic scan of the chemical area and savings of chemicals other than water.
Three ternary samples located in the biphasic region were prepared by mixing HDec and N4444Cl, performing the heating process and then adding the total amount of water in a single operation instead of successive additions of limited amounts. The mixture was then subjected to the general thermostatic protocol in order to check any possible influence of the successive water additions onto their final state. No difference could be observed. It should be noted that, in many cases, the time needed for phase disengagement in the biphasic area was quite long, a phenomenon which was already pinpointed [34].
In order to determine two tie-lines of H2O/HDec/N4444Cl, two biphasic samples have been prepared and their upper and lower phases have been separated and weighted. The volumes of each phases have been measured, thus densities have been derived. pH values of the upper and lower phases have been measured with a pH-meter (WTW, pH 3110 series) and chloride concentrations have been measured with a Cl−-specific electrode (Thermo Scientific, chloride half-cell Orion 9417SC and reference cell). A linear calibration has been obtained with NaCl aqueous solutions in the range 5 × 10−3 M/1 M and checked with N4444Cl aqueous solutions. Aliquots of the lower phase have been measured, after appropriate dilution with ultra-pure water. The chloride content of the upper phase could not be measured because water dilution to reach the calibration range induces the immediate formation of a white solid, which has not been identified, but might be a mixture of HDec and N4444Cl. NMR spectra were recorded on a Bruker AVANCE III HD 400 MHz spectrometer equipped with a 5 mm BBO probe allowing the observation in 1D (1H and 13C) for 1H at 400.15 MHz and 13C at 100.12 MHz and in 2D with homonuclear correlation spectroscopy (1H–1H COSY) and Heteronuclear Single Quantum Coherence Spectroscopy (1H–13C HSCQ). The chemical shift (δ) is measured in the unit of part per million (ppm). The coupling constant (J) is expressed in Hertz (Hz). For calibration, an inner tube containing d6-DMSO is used. (δH: 2.50 ppm and δC: 39.52 ppm). Multiplicities are reported as follows: t = triplet, sext = sextuplet and m = multiplet. Pure HDec and pure N4444Cl dissolved in d-DMSO were measured as blank samples. Assignments are (see Figure 1 for C numbering):
Numbering of carbons for NMR.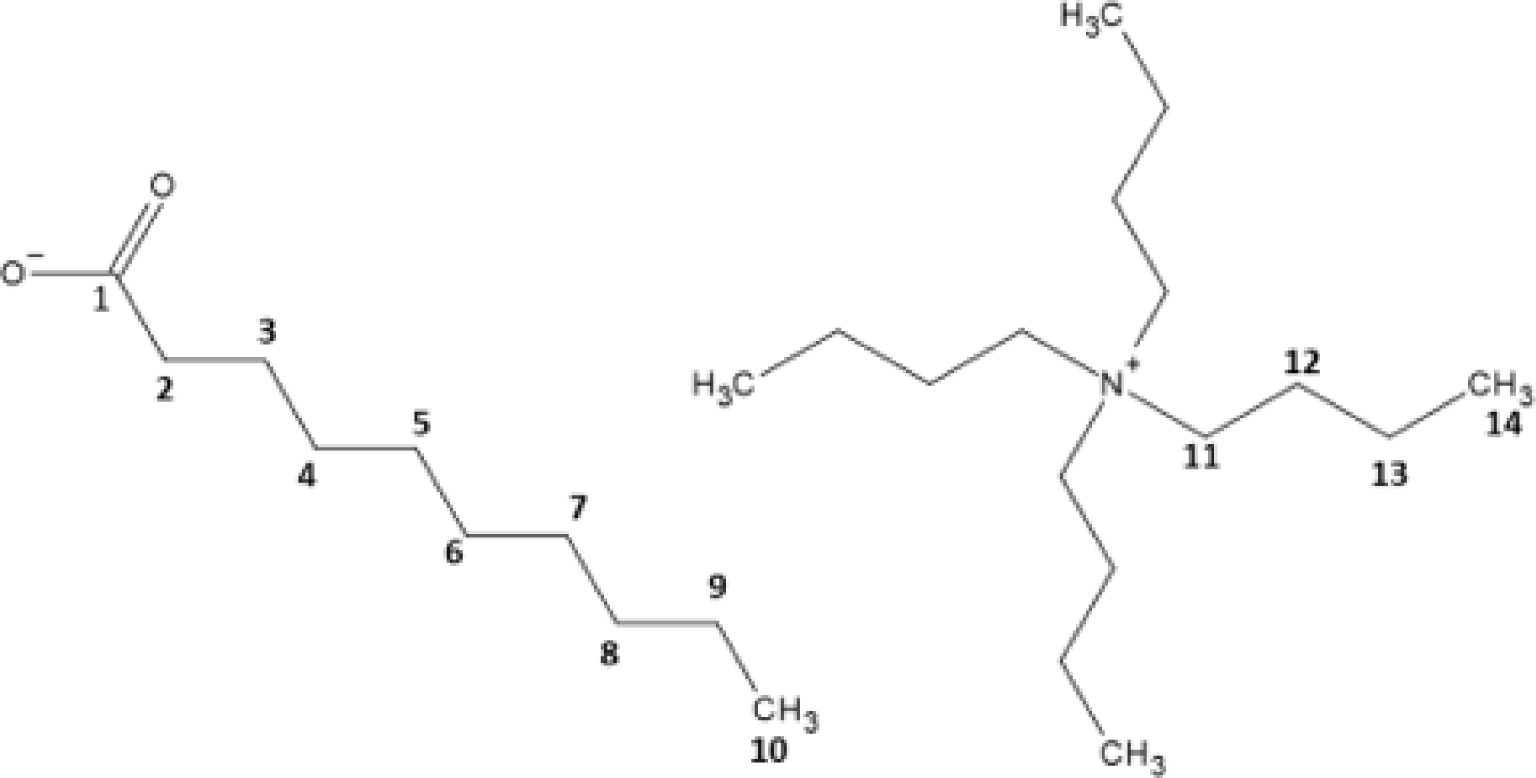
Lower phase: RMN 1H (400.15 MHz): δ (ppm) = 2.63 (m, 8H, H11), 1.08 (m, 8H, H12), 0.81 (sext, 8H, J = 7.2 Hz, H13), 0.39 (t, 12H, J = 7.3 Hz, H14). RMN 13C (100.12 MHz, DMSO-d6): δ (ppm) = 57.2 (C11), 22.2 (C12), 18.3 (C13), 12.1 (C14).
Upper phase: RMN 1H (400.15 MHz): δ (ppm) = 2.95 (m, 8H, H11), 1.90 (t, 2H, H2), 1.31 (m, 8H, H12), 1.22 (m, 2H, H3), 1.04 (m, 8H, H13), 0.93 (m, 12H, H4, H5, H6, H7, H8, H9), 0.60 (t, 3H, J = 6.95 Hz, H14), 0.53 (t, 3H, J = 6.6 Hz, H10). RMN 13C (100.12 MHz, DMSO-d6): δ (ppm) = 175.1 (C1), 57.4 (C11), 33.3 (C2), 31.1 (C4), 28.7 (C8), 28.6 (C7), 28.5 (C5), 28.4 (C6), 24.1 (C3), 22.9 (C12), 21.8 (C9), 18.7 (C13), 13.0 (C10), 12.6 (C14).
All data have been acquired at T = (25 ± 1) °C. Uncertainties on volumes are estimated at ±0.1 ml, those on densities at ±3% and those on chloride concentrations within 1%. The relative error on mass balance is within 1.5%.
3. Main characteristics of the accepted three classes and definitions used in this work
For each category, the wealth of data already published under the keywords “organic solvent” or “ABS” or “DES” liquid–liquid extraction systems covers a very broad variety of mixtures, which definitions are sometimes not clear or may differ from one publication to the other, especially in the case of “DES” (see Section 3.3 below). Therefore, a summary of the most commonly accepted compositions will be first given, then the general accepted characteristics of such systems will be recalled and finally, the definitions to be considered in this work will be given.
3.1. Class #1: LLES with organic solvents and water
This category is probably the most investigated one and has thus benefited from several improvements in terms of composition. Basically, these systems comprise water plus an organic solvent, often called the diluent, in which an extractant (β-diketones, crown-ethers, phosphine oxides, aliquat etc.) is eventually dissolved. Many papers concern organic molecular solvents, such as n-hexane, benzene, dichloroethane, chloroform etc. but the rise of organic compounds called hydrophobic ionic liquids (ILs) has broaden the field [37, 38, 39, 40, 41, 42, 43]. In this work, ILs will be defined following their most common definition, as salts displaying a melting temperature below or equal to 100 °C. As the organic phase is contacted with water, and depending on temperature and proportions, a biphasic state is obtained. In the simplest cases, the extractant is liquid at the chosen working temperature and can thus be used in its pure form. Some adjuvants to the organic phase (for example, long-chain alcohols) can be added up to a few percent, to control viscosity or avoid the formation of third phase [44]. A second extractant can also be envisioned, to induce synergistic extraction [45, 46, 47]. Furthermore, many starting aqueous phases are acidic. This comes from the need to prevent the undesired hydrolysis/precipitation of the metal ions of interest in the case of fundamental studies and from the leaching/dissolution step performed prior to liquid extraction in the case of applied studies dealing with real industrial wastes samples. In some case, complexing agents can be added to the aqueous phase as masking agents to enhance separation factors [48]. All these modifications result in an increased number of chemical components in the system. However, at minimum, these LLES are composed of two different chemicals, i.e. water and an organic liquid compound but, most of the time, they are composed of three or four chemicals. The common property of these liquid mixtures is the claimed immiscibility of the organic part with the aqueous phase under the investigated conditions.
In this work, in order to encompass as many systems as possible, any mixture of water plus one organic compound, either molecular or ionic, will be considered as belonging to category #1, provided that there are some temperature and proportion conditions under which the organic solvent is not fully miscible with water and regardless of any additional compounds this mixture may contain (extractant, synergist, acid, adjuvant, masking agent etc.). This definition is chosen in order to include LLES involving hydrophobic ionic liquids as diluents.
3.2. Class #2: LLES as ABS
Giving a clear and precise definition of such systems is not obvious and a previous definition suggested by the author of this paper a few years ago finally appears unsuitable in the context of this work [49].
According to the commonly accepted denomination, ABS systems (also named aqueous two-phase systems, ATPS) are ternary mixtures which can be composed of (i) two polymers plus water [50, 51], or (ii) a polymer, a salt plus water [52, 53, 54, 55] or (iii) two salts, one being an ionic liquid (IL) plus water [56, 57, 58, 59]. Note that the term “salt” here includes mineral acids (HCl, HNO3, H2SO4 etc) [60, 61] as well as metal salts [62]. The polymers and the salts are said to be individually highly soluble in water but a biphasic state appears as the three compounds are mixed together in certain proportions and depending on temperature. Similarly to the case of category #1, additives can be used, such as inorganic salts, ILs or extracting agents [63, 64].
Under the biphasic state of the system, the two phases are termed as salt-rich and polymer- (or IL-) rich, respectively, but authors also stress the fact that water partitions in both phases, thus the name of aqueous biphasic systems. In fact, all constituents of ABS can be found in both the upper and the lower phase, with different proportions, depending on initial amounts and temperature. Exact compositions of the upper and lower phase can be deduced from the tie-lines and the binodal curve.
In this work, category #2 includes liquid mixtures composed of three chemicals at minimum, one being water, two others being absolutely necessary together for the appearance of a biphasic state, when contacted with water, under some conditions of temperature and proportions. As a consequence, binary mixtures of water and one single thermomorphic chemical, such as betainium bis(trifluoromethylsulfonyl)imide [65], or C1C4imFeCl4 [66] are not included in category #2. They however fit into category #1.
3.3. Class #3: LLES with DES and water
There is currently a healthy debate about the definition of what a DES is. In the pioneer works of Abbott et al. [67, 68] in 2003 and 2004, no precise definition can be found but, in 2017, Abbott and co-workers [69], citing these two first publications, stated that “DES are mixtures of Lewis and Brönsted acids and bases which produce low melting point systems due to complex formation”. Other definitions to be found in the literature comprise “mixtures of two or three safe components that are able of associating with each other through hydrogen bond interactions” [70], or “the mixture of an H-bond donor and an H-bond acceptor” [71]. The discovery and subsequent use of hydrophobic DES [30, 32, 33, 34, 72, 73, 74], for liquid–liquid extraction did not help in fixing a more precise definition of DES. Martins and co-workers [75] recently noted that “Due to the absence of a strict and clear definition of what a deep eutectic solvent is, this term is often abused” and added that “a ‘deep eutectic solvent’ should be defined as a mixture of pure compounds for which the eutectic point temperature is below that of an ideal liquid mixture”. This is in perfect agreement with another recent publication [76] stating that the term deep “should only be used for systems showing melting points significantly below ideal predictions”. In a recent review paper [77], Hansen and co-workers also strongly support this definition. This thermodynamic definition based on the discrepancy between experimental melting points and ideal predictions is perfectly unambiguous, precise and clear and should prevail. However, so far, the vast majority of papers claiming liquid–liquid extraction with “DES” do not measure the liquid–solid equilibrium and do not compare to the prediction of the ideal case. Therefore, considering the very broad (mis)use of the term DES, and in order to encompass as many systems as possible, in the meantime better, it is decided in this work to include in category #3 “real” DES, (i.e. complying with the thermodynamic definition above), and all systems considered as potential LLES, containing at minimum water plus a mixture of two compounds and termed as hydrophobic DES by their authors, even without experimental proof. The additional criterion of chemical stability will be also considered, in order to exclude the numerous systems possibly experiencing the esterification of alcoholic and carboxylic functions and thus long-term instability [78].
The general characteristic of these mixtures is the formation of a homogeneous clear liquid phase under some composition and temperature range plus, when contacted with water, the formation of a biphasic state, thus allowing extraction.
4. Are the claimed specific characteristics of these categories really meaningful?
The major discriminating claimed aspects of these three categories are based on the chemical nature of their constituents and some of their general physico-chemical properties. Organic solvents are found in category #1 and their immiscibility with aqueous phases is the main characteristics put forward. By contrast, the presence of water within the two phases of the biphasic state appears a distinctive feature of category #2, while the new-born category #3 apparently will build its reputation on the use of H-donors and H-acceptors although it may turn differently. In the following sections, these arguments, brought in favour of three distinct classes, will be discussed.
4.1. Arguments based on composition and nature of the constituents
Organic compounds are to be found in all cases throughout the three categories. Apart from category #1, for which it is assumed to be a distinctive feature (for both the diluent and the extractant, if ever) polymers and ionic liquids are actually organic compounds. Similarly, weak organic acids, H-acceptors and H-donors of so-called hydrophobic DES are also organic compounds. In fact, to the best of my knowledge, there is no mixture called a hydrophobic DES and composed of purely inorganic compounds. Furthermore, ionic liquids can be found in all categories. As already pinpointed, ILs can act as extractants whenever dissolved in molecular solvents [79] or can act as diluent and/or extractants [80, 81, 82, 83], they have brought a fruitful impulse to ABS [56] and some of them, acting as H-acceptors, enter in the composition of several DES [84, 85, 86]. Since ILs and, on a more general perspective, organic compounds, can be found in all three categories, it is reasonable to question whether a categorization based on such a chemical aspect really makes sense because using non-disjoint categories to classify systems is a risky method.
4.2. Aqueous immiscibility versus aqueous solubility
First, the terms “insoluble/soluble”, or “poorly/highly soluble”, do not offer the required quantitative basis for categorisation and, given alone, are merely a matter of personal feelings. Second, all organic solvents in category #1 are more or less soluble in water. Benzene is soluble in water to ca. 1.79 g⋅l−1, i.e. 2.3 × 10−2 M (at 25 °C), and chloroform in water amounts to 3.81 g⋅l−1 i.e. 3.2 × 10−2 M (at 25 °C). Reversely, the water solubility in organic solvents is not nil either and these mutual solubilities are well-known to experimentalists, who often counter balance this double phenomenon by equilibrating aqueous and organic phase prior to the addition of the metal ions to be extracted. These mutual solubilities are thus reminiscent of those taking place in category #2 and being the characteristic of this category. Similarly, in the case of category #3, there are now quite a few papers evidencing the loss of the DES components in the aqueous phase to different extends, thus modifying the DES composition and properties [70, 85, 87, 88]. This is in line with Dwamena’s remark, in a recent review on DES [71], stating that “since the hydrophobicity of hydrophobic DES is relative, the distinction between hydrophilic and hydrophobic DES is sometimes confusing and hence a comprehensive guideline for calling DES hydrophobic should be established”.
On another hand, some constituents of hydrophobic DES have water solubilities close to that of organic solvents. For example, van Osch and co-workers, who were the first to present hydrophobic DES, investigated systems based on H2O, decanoic acid (2 moles) and six different ammonium-based ILs (1 mole), in particular N4444Cl but also N8888Br [34]. Decanoic acid has a solubility in water of approximatively 0.15 mg⋅g−1, i.e. 8.7 × 10−4 M (at 20 °C), a value of the same order of magnitude as that of a very well-known organic solvent such as n-hexane : 9.5 mg⋅l−1, i.e. 1.1 × 10−4 M (at 25 °C). For any of the six mixtures they studied, the authors always used the term DES while these could be viewed as category #1 members, where decanoic acid is the diluent and where the IL is the extractant. In another publication, it is emphasized that “Ideally, a fully separated system, with cross contamination as low as possible, should be achieved with a pure DES phase in contact with a pure water phase, separated by a sharp interface” [72]. This remark could be applied to any system of category #1, the only difference being that DES always require two compounds, while organic molecular solvents alone comply with this remark.
Turning the argument around, for the system H2O/HDec/N4444Cl [34], also investigated in this study, it appears that the water concentration in the organic phase reaches ca. 7%, while the solubility of N4444Cl in water equals ca. 35% of the total amount used, a phenomenon ascribed by the authors to the “high solubility” of N4444Cl in water. Altogether, these facts are indicative of water, IL and decanoic acid partitioning at varying degrees among both phases of the system, so the tag “ABS” could have been used instead of (hydrophobic) DES. Actually, closely related systems, composed of H2O/N4444Cl/K3PO4 [89], or H2O/N8888Br/HNO3 [60] are termed ABS by their first investigators. Similarly, in the systematic search for new hydrophobic DES, van Osch and co-workers eventually obtained DES having “a high water content”, above 20 wt% and up to ca. 34 wt% [72]. These values would not seem abnormal for ABS systems.
Again, through these examples, it is shown that systems may well belong to two or three categories, therefore ruling out the use of these categories. In fact, the mutual solubilities of each constituents through the three categories under discussion simply differ from one system to the other on a quantitative basis, but are not different in nature, because this is always the same phenomenon. At minimum, a clear distinction should be based on a quantitative scale, fixing precise limits within which compounds should be qualified as “soluble”, “moderately soluble” or “insoluble”. As long as this is not the case, categories based on such vague terms appear of dubious use and interest.
5. Organic solvents versus ABS versus DES: Much ado about nothing or fruitful harmonisation?
As deduced from the above discussion, the usefulness of three categories that can welcome the same systems is under question. It would thus be reasonable to gather all these systems in one unique class, which could be named “aqueous liquid/liquid systems”. “Aqueous Two-Phase Systems” (ATPS) could also be a possibility. The important point is to highlight the ubiquitous presence of water in all these systems and the existence of two distinct phases. Time will tell which name will be favoured. The author of this deliberately somewhat polemical paper is well aware of the fact that she herself has used the terms she criticizes today. Part of the reasons might be found in the attractivity papers tagged with “ABS” or “DES” experience at the moment. Maybe it is not too late to fight against such a specious argument, just as the DES community is doing, now fighting against the excessive use of “deep” for systems that are simply eutectic ones [75, 76].
While pointing out the redundancy of the three categories is a necessary task, an even better attitude is to highlight the strong link between all these systems. To this aim, a quantitative harmonisation is proposed in the following. Contrary to what is commonly accepted, the grand unifying aspect of these systems is found in the mutual solubilities of all their constituents.
Phase diagrams of ternary systems can be rather complicated, possibly including more than two different phases, some of them being liquid or solid. In view of liquid/liquid extraction, the presence of a third solid phase is often, but not always, considered as undesirable and a major disadvantage [44, 90]. In view of liquid/liquid extraction, the “useful part” of a phase diagram is limited to the area where two liquid homogeneous phases are found. Describing the system through partial phase diagram including only the biphasic (liquid/liquid) and the monophasic (liquid) areas is therefore common practice in the ABS community, although some more comprehensive diagrams can be found too [91, 92]. As an example of the usefulness and interest of this proposed common quantitative descriptor, Figures 2 and 3 display the phase diagrams and some tie-lines, as obtained from literature data, for the systems H2O/HNO3/TBP and H2O/Na2SO4/PEG-4000, where PEG-4000 stands for polyethylene glycol of average molar mass 4000. The third phase diagram, for H2O/HDec/N4444Cl at 25 °C (Figure 4) was obtained according to the experimental protocol described in Section 2. These three systems have been taken as archetypal examples of categories #1, #2 and #3, respectively. Table 1 displays the structure of the chemicals under focus in this paper.
Binodal curve (blue symbols) of the ternary system H2O/HNO3/TBP, from references [93, 94]. A–A′, B–B′ and C–C′ are tie-lines (orange symbols and lines, see Section 5). For S1 and S2 chemical composition, see Table 2.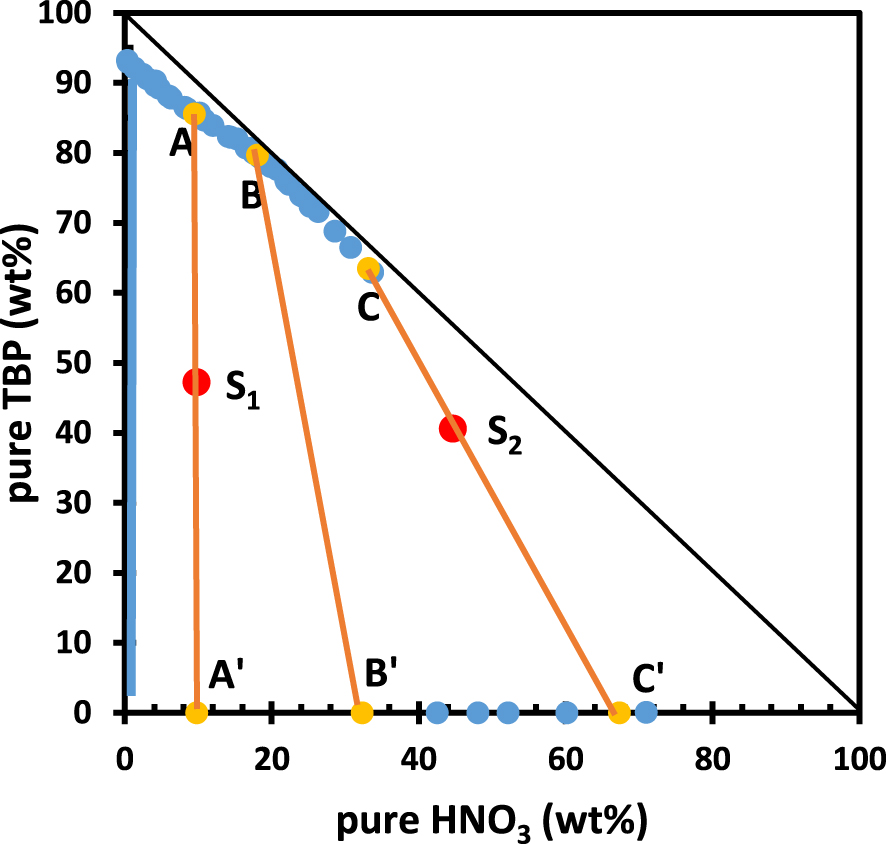
Binodal (blue symbols) and some tie-lines (orange symbols and lines) of the system H2O/Na2SO4/PEG-4000 at T = 25 °C. Data redrawn from Ref. [92].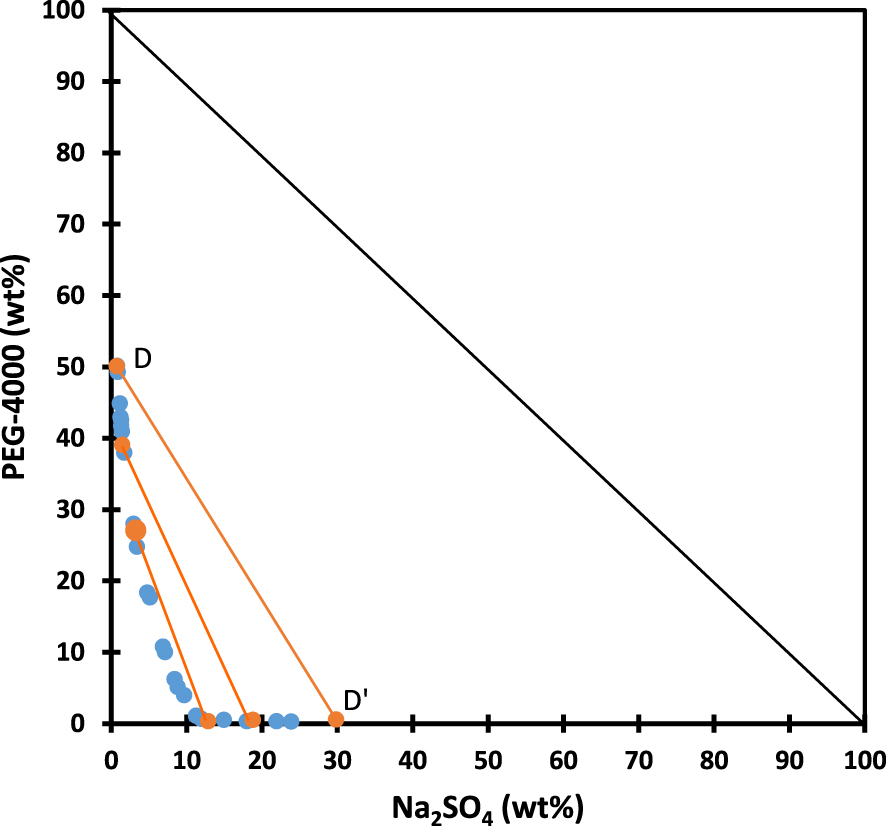
Chemical structures, names and acronyms of the compounds under focus in this work
| Name (acronym) | Chemical structure |
|---|---|
| Tributyl phosphate (TBP) | 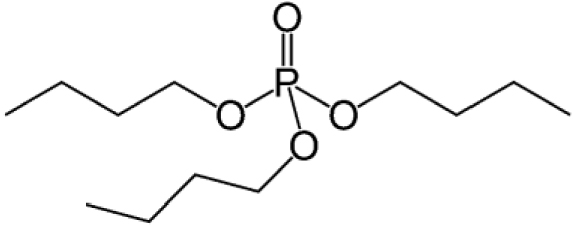 |
| Polyethylene glycol (PEG) |  |
| Tetrabutyl-ammonium chloride (N4444Cl) |  |
| Decanoic acid (Dec) |  |
Phase diagrams of ternary systems can be displayed in a triangular or an orthogonal plot. These two modes of graphic representation offer exactly the same quantitative information. In the ABS community, the orthogonal mode is the most widely used [56], therefore it was also adopted in this work. However, the corresponding triangular representations can be found in the supplementary materials (Figures S1–S3).
Data for the system H2O/HNO3/TBP have been plotted in units of weight percent (wt%) taking advantage of literature data expressed in mole [93] or in g⋅l−1 [94]. Data concerning TBP in the range 60–95 wt% were collected at T = 25 °C [93] while the data selected for TBP amounts below 10 wt% are those collected at T = 22 °C [94], because the number of data point is more important. To the best of our knowledge, there are no other data of this kind for H2O/HNO3/TBP. Values (in wt%) for this graph can be found in the ESI (Table S1). The binodal curve thus plotted is in general agreement with the triangular representation found in [95] (T = 25 °C) but in this last paper, the data are not in an easily usable form. In Figure 2, the x-axis corresponds to aqueous solutions of nitric acid, without TBP and these binary mixtures are always monophasic. Actually, data close to the HNO3 axis are slightly above the x-axis (see ESI). By contrast, the y-axis, corresponding to binary mixtures of TBP and water (no HNO3) mainly belongs to the biphasic region of the phase diagram, because the solubility of TBP in pure water is of the order of 0.39 g⋅l−1, i.e. 1.46 × 10−3 M or 0.04 wt% (at T = 25 °C) [94]. This is symbolised by a blue vertical line in Figure 2. The straight lines A–A′, B–B′ and C–C′ in Figure 2 correspond to three tie-lines. For any sample composition, S, belonging to tie-line A–A′, the compositions of the lower and upper phase are that of points A and A′, respectively, while the volume ratio of the two phases is equal to the ratio of the lengths A′–S to S–A.
| TBP (g) | Pure HNO3 (g) | H2O (g) | TBP (wt%) | Pure HNO3 (wt%) | H2O (wt%) | |
|---|---|---|---|---|---|---|
| S1 | 0.89 | 0.184 | 0.81 | 47.24 | 9.77 | 42.99 |
| S2 | 1.38 | 1.52 | 0.50 | 40.59 | 44.71 | 14.70 |
Figure 2 shows that most of the phase diagram corresponds to a biphasic state at T = 25 °C, which is a well-known practical result in the nuclear field. As the biphasic state is largely the dominating case, one might think that these data are almost useless by arguing that almost any proportions of water, TBP and nitric acid will give a biphasic state, thus allowing extraction. However, a close look at the tie lines brings some rather interesting information. The tie-lines are almost vertical close to the y-axis (slope is infinite, see tie-line A–A′), while they tend to be inclined at 45 degrees as the mass percentage of HNO3 increases (see tie-line C–C′). Note that tie-lines are not parallel either in the triangular representation (see Figure S1). In Figure 2, points S1 and S2 (see Table 2 for chemical compositions) correspond to two different H2O/HNO3/TBP mixtures, belonging to tie-lines A–A′ and C–C′, respectively. S1 has been chosen as to be as representative as possible of a usual practical case of liquid–liquid extraction with TBP, i.e. identical volumes of aqueous and organic phase prior to contact (ca. 0.9 ml in the case chosen here) and “moderate” HNO3 concentration in the starting aqueous phase, i.e. 3.25 M. S2 is representative of a less frequent case in the literature, based on similar volumes of the aqueous and organic phase prior to mixing and 17 M HNO3 but is similar in lower values to studies involving fuming nitric acid (fuming nitric acid is ⩾ 20 M) [96].
For both samples S1 and S2, the total amount of TBP to be found in the lower phase is below 0.05 wt%, which means in turn that almost 100% of the TBP is present in the upper phase. In other words, the partition of TBP among the two phases is exceedingly in favour of the upper phase, for both S1 and S2. This is not true, however, regarding water and nitric acid. In fact, TBP represents only 85.6 wt% and 63.5 wt% (points A and C in Figure 2, respectively) of the total mass of the upper phase. This means that the upper phase has been massively loaded with nitric acid (ca. 33 wt% for point C) and the rest (ca. 3.5 wt%) being water. The question of water and nitric acid extraction by TBP has been, and still is, the subject of very intensive research [97, 98] which basis can be found in the phase diagram as displayed in Figure 2, although, of course, this diagram does not give any insight into the exact nature of the HNO3-water-TBP complexes.
In Figure 3, data for the system H2O/Na2SO4/PEG-4000 at T = 25 °C are redrawn from literature [92], again using weight percent units.
The ternary mixtures of H2O, Na2SO4 and PEG of different masses have been quoted as ABS by several authors already long ago [51, 52, 53, 92, 99, 100, 101, 102] and they belong to category #2. As for H2O/HNO3/TBP, the tie-lines are not parallel to each other in both orthogonal and triangular plots. As the binodal comes very close to the x-axis, regardless of the overall composition, PEG-4000 is almost not present in the lower phase, thus behaving like TBP. From the tie-lines known from literature (see Figure 3), one easily deduces that the upper phase may also contain large amounts of water with a rather limited amount of Na2SO4. For example, for tie-line D–D′ the upper phase composition is ca. 50 wt% of PEG-4000 and 50 wt% water, with less than 1 wt% of Na2SO4.
As for category #3, the objective of the limited experimental campaign of this work was simply to explore the phase diagram of the ternary H2O/HDec/N4444Cl system at 25 °C, i.e. determining part of the binodal and two tie-lines. Comparison with other works is very limited because the only information already published in the literature on the binary system HDec/N4444Cl or on the ternary system H2O/HDec/N4444Cl is that provided by van Osch et al. [34]. van Osch and coworkers studied a single HDec/N4444Cl ratio (2:1, in mole) and indicated that this 2:1 HDec/N4444Cl sample (no water added) has a freezing temperature at ca. −12 °C [34]. However, it cannot be inferred from this single value that this is the composition displaying the lowest melting temperature of the binary system HDec/N4444Cl and no comparison with the ideal thermodynamic value was provided. The HDec/N4444Cl mixture has nevertheless been tagged as a DES by these authors [34].
Binodal (blue symbols) for the system H2O/HDec/N4444Cl at T = 25 °C. Tie-lines (orange symbols and lines) for the two samples described in Table 3. Points Svoi, Svom and Svof: subscript “vo” is for van Osch; subscripts i, m and f are for initial (no water added, see [34]), middle (ca. middle in between Svof and the tie-line) and final (as much water as in [34]).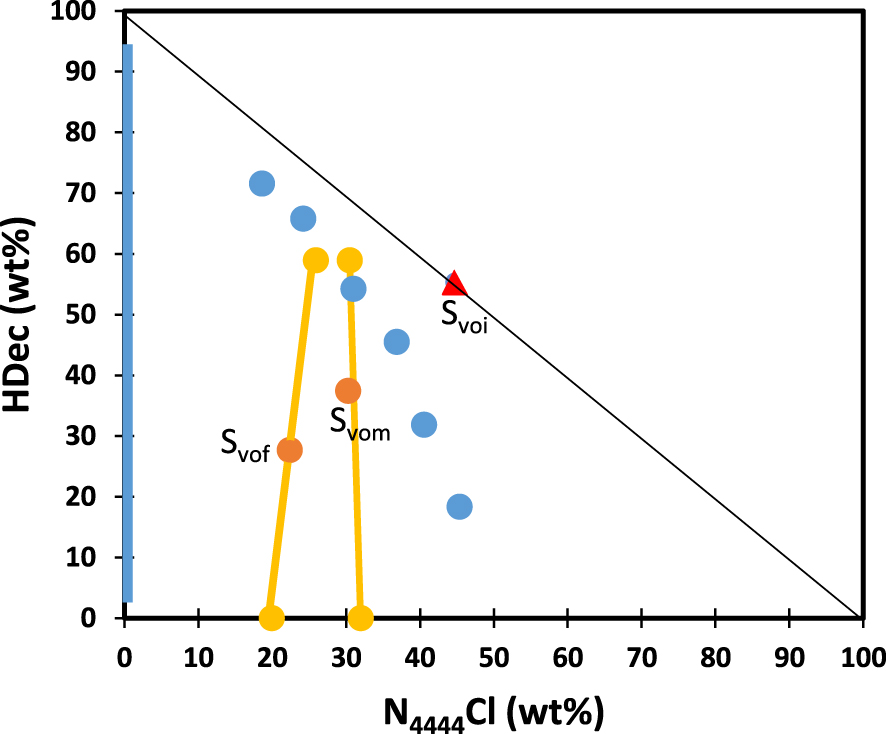
The sample prepared during this work with this 2:1 HDec/N4444Cl molar composition is actually a clear monophasic liquid at T = 25 °C (see ESI, Table S2 and point Svoi in Figure 4, where subscript vo stands for van Osch and subscript i for initial) but no attempt to determine its melting temperature was made. Some other limited information about the binary phase diagram could also be obtained from the experimental protocol used in this work. According to data from the producers or from data security sheets, the melting temperature of N4444Cl is 113 °C and that of decanoic acid is 31 °C. By choosing T = 25 °C, i.e. 6 °C below the melting temperature of decanoic acid, it can be expected that some binary compositions with high amounts of N4444Cl or HDec would be solid at T = 25 °C. This is actually the case for data points S3 (HDec: 86.17 wt%; N4444Cl: 13.83 wt%), S4 (HDec: 21.26 wt%; N4444Cl: 78.74 wt%) and S5 (HDec: 9.98 wt%; N4444Cl: 90.02 wt%) which are in solid form at T = 25 °C (see Figure S4 in ESI). Then, van Osch and co-workers prepared a single ternary mixture, by adding a water mass equal to that of the 2:1 HDec/IL binary mixture and they obtained a biphasic system at T = 25 °C. The point tagged Svof in Figure 4 (subscript f stands for final) corresponds to their composition and is in a biphasic state.
The binodal displayed in Figure 4 (T = 25 °C) has been obtained according to the experimental protocol described in Section 2, and all values are gathered in the ESI (see Table S2 and Figure S4 which illustrates the biphasic liquid, monophasic liquid and solid samples obtained). Owing to the very low solubility of HDec in pure water, almost all the y-axis is in the biphasic state and this is symbolised by the solid vertical blue line in Figure 4. By contrast, a large part of the x-axis corresponds to a monophasic liquid state, due to the large solubility of N4444Cl in water. The limit of the biphasic area should be close to the x-axis but above it.
The two tie-lines associated to samples Svom (subscript m stands for middle, i.e. in between Svof and the tie-line) and Svof, were determined as follows (see Table 3 for sample compositions). First, the pH values were measured in the upper and lower phases (Table 3). In these ternary samples, H+ comes only from HDec dissociation. Although the upper and lower phases can be considered as rather acidic as compared to ultra-pure water, these pH values nevertheless correspond to H+ amounts that are at least ca. 240 times lower than the initial HDec amounts so one can safely assume that decanoic acid is only present in its associated form. This is consistent with its weak acidic properties. Second, NMR data for the lower phases could not evidence the presence of HDec (see Figure S5 in the ESI). Consequently, the contributions (in wt%) of HDec to the lower phases were arbitrarily set to zero. Third, measurements of the chloride ion concentrations in the lower phases and mass balances were used to derive the upper and lower phase compositions (Table 3). The molar ratio between HDec and N4444Cl in the upper phase, RHDec/N, can thus be calculated and compared to the initial 2:1 value.
Independently from these measurements and subsequent calculations, RHDec/N can also be obtained solely by using the NMR data of the upper phases. In their work, van Osch et al. indicated that a quantitative NMR analysis is hampered by the partial overlap of the decanoic and ammonium patterns and by overlap of the water and ammonium peaks [34]. This was also observed in the experiments of this work but the ambiguity could be resolved by 2D-NMR experiments (see Figures S6 and S7 for sample Svof). Table 3 gathers the RHDec/N values obtained from NMR data only, and by use of the chloride measurements, for the two samples of this study. As can be seen, the RHDec/N values obtained from the two methods agree rather well. These results allow us to draw the two tie-lines in Figure 4. As can be observed, the points corresponding to the upper phases, lower phases and preparation samples are pretty well aligned as expected, and in good agreement with the binodal curve, as expected too.
Composition (in wt%) of the two samples prepared in view of tie-line determination. Compositions (wt%) of their upper and lower phases, pH, densities and RHDec/N ratio (see text)
| Sample 1 | Sample 2 | |
|---|---|---|
| Preparation | HDec/N4444Cl/H2O | HDec/N4444Cl/H2O |
| 37.4/30.3/32.3 | 27.7/22.4/49.9 | |
| Upper phase | HDec/N4444Cl/H2O | HDec/N4444Cl/H2O |
| 58.9/30.5/10.6 | 58.9/25.9/15.2 | |
| pH = 2.12; d = 0.91 | pH = 2.44; d = 0.91 | |
| RHDec/N from NMR: 3.0 | RHDec/N from NMR: 3.8 | |
| Lower phase | HDec/N4444Cl/H2O | HDec/N4444Cl/H2O |
| 0.0/32.0/68.0 | 0.0/19.9/80.1 | |
| pH = 1.74; d = 0.98 | pH = 2.05; d = 0.98 | |
| RHDec/N = 3.1 | RHDec/N = 3.7 |
This phase diagram is rather similar to that of the system H2O/HNO3/TBP (Figure 2). As TBP, HDec is almost not present in the lower phase, while N4444Cl has a significant contribution to the total mass of the upper phase, as HNO3 has. Furthermore, one of the two tie-lines experimentally obtained for H2O/HDec/N4444Cl is vertical, a fact also observed for H2O/TBP/HNO3 (Figure 2).
In conclusion, as can be seen from Figure 4, a rather easy-to-perform set of experiments demonstrates that the mixture of a “hydrophobic DES” and water can be treated as an ABS in terms of phase diagram (binodal and tie-lines). An important information derived from Figure 4 is that HDec and N4444Cl partition to different extends in the upper and lower phase so that the initial molar ratio of 2:1 is lost as soon as water is added (see RHDec/N ratio in Table 3). This was already pinpointed by van Osch and co-workers who evidenced the leaching of N4444Cl toward the lower phase [34]. This DES cannot be considered as one single compound, and this is a general phenomenon for “hydrophobic DES” [88]. Therefore, there is no reason to consider that ABS and “hydrophobic DES” plus water are different enough to be classified in two different categories of LLE systems. Similarly, there is no reason to insert H2O/TBP/HNO3 and H2O/HDec/N4444Cl in two different LLE categories, as HDec behaves as TBP, while N4444Cl can be satisfactorily compared to HNO3 in terms of biphasic behaviours, as illustrated in the similarities observed in the binodals and tie-line data of the two systems.
6. What about usage, applications and usefulness?
LLES, whatever their composition, should be examined under the light of their potential industrial applications. To this end, industrial requirements and regulations should be considered. Cost and efficiencies are usually the main criteria for industrial partners while toxicity to the bio and geosphere is the subject of many regulations, such as REACH for EU. Clearly, cost and toxicity cannot be inferred from the phase diagram. Furthermore, they are variable criteria because the cost of energy and chemicals, especially those obtained from fossil resources, are highly volatile data, while regulations evolve over time. This aspect cannot be discussed at length in this paper, but it is important to bear in mind that industries may be forced to use more and more biosourced compounds in the future.
Another important point for industrial applications is the possible losses of some compounds through the mutual solubilities discussed above. This is quantified thanks to the tie-lines. The phase diagram of H2O/Na2SO4/PEG-400 (Figure 3) is a very good candidate to avoid cross-pollution, because the borders of the biphasic region are very close to the x and y-axis and tie-lines are sufficiently inclined to almost reach the x and y axes. By comparison, the systems in Figure 2 (H2O/HNO3/TBP) and Figure 4 (H2O/HDec/N4444Cl) are not as favourable from this point of view. However, surprises can appear as metal extraction is performed, because it has been shown that some metal ions have a very strong influence on the shape of the phase diagram [36].
7. Conclusion
By comparing the phase diagrams for three archetypal liquid–liquid extraction systems usually considered as very different because being based on a molecular organic solvent (H2O/HNO3/TBP), or classified as an ABS (H2O/Na2SO4/PEG-400) or tagged as a hydrophobic DES (H2O/HDec/N4444Cl) this paper evidences the underlying identical phenomenon of mutual solubilities at work in each of them. In this sense, there is no obvious need for three different categories, which are not properly and unambiguously defined yet. In fact, rather than separating LLE systems into three supposedly distinct categories, it would certainly be a more fruitful perspective to acknowledge that “ABS” and “hydrophobic DES” dramatically extended the number of systems that can be used for extraction of metal ions. This proposed unification of concepts is certainly inspiring.
The systems of concern in this work all contain water, as this is the most usual solvent in which metal ions may be found, once the basic primary operations such as grinding have been applied to ores or technological wastes. Water is also ubiquitous in the numerous liquid wastes generated by industries of various kind. However, one can assume that mutual solubility is not a phenomenon restricted to water-containing systems. Actually, new trends in extraction/separation of metal species are rapidly emerging, as exemplified by the recent results of successful leaching of several compounds from grinded batteries by use of a choline chloride/ethylene glycol mixture [103], leaching of metals from NdFeB magnets by a choline chloride/lactic acid mixture [104] or leaching of In and Sn from zinc flue dust by use of a choline chloride/oxalic acid dihydrate mixture [10]. These results are complementary to those already obtained with non-aqueous systems for liquid–liquid extraction of metal ions and named as solvometallurgy [105]. Several non-aqueous extraction systems have actually already been studied in the literature, for example two ILs [106], one IL and one alcohol [107] cyclohexane/choline chloride/ethylene glycol [69] or ethylene glycol versus dodecane plus Cyanex 923 as extractant [108]. In the latter study, “it was found that about 24.5 g⋅l−1 of ethylene glycol was co-extracted into 1M Cyanex 923”, thus supporting our general view of mutual solubilities, whatever the system studied. Another confirmation of the ideas discussed in this work could be found in three liquid phase systems, as reviewed recently in an excellent paper [109]. For example, in the mixture aliphatic hydrocarbon/CH3CN/NaCl in water, “the water content in the middle phase is difficult to control (…), and some of the polar organic solvent is also driven into the (bottom) aqueous phase” [109]. Four liquid phase systems are not left out as exemplified already more than ten years ago for pentane/[P66614][NTf2]/water/[C2mim][NTf2] where mutual solubilities have been evidenced with each ion behaving independently [110]. For all these systems of increasing complexity the precise knowledge of mutual solubilities is mandatory, because such values are important parameters to be considered, as they highlight crossed pollutions and losses of chemicals that can have an environmental impact and should therefore not be neglected anymore [111]. To this aim, the determination of phase diagrams is an essential first step.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgements
Discussions with Dr. Jean-Michel Andanson about DES and the help of Laure Cointeaux for the NMR study are gratefully acknowledged. This work has been performed under the frame of the REFINA European project.




 CC-BY 4.0
CC-BY 4.0